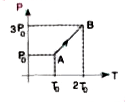
Pressure versus temperature graph of an ideal gas is as shown in figure. Density of the gas at point A is ρ0. Density at B will be:
 Solution in Telugu
Solution in Telugu
34ρ0
32ρ0
43ρ0
2ρ0
The correct Answer is:B
Step by step video, text & image solution for Pressure versus temperature graph of an ideal gas is as shown in figure. Density of the gas at point A is rho_0. Density at B will be: by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- THERMAL PROPERTIES OF MATTERBOOK - AAKASH SERIESCHAPTER - THERMAL PROPERTIES OF MATTEREXERCISE - EXPANSION OF GASES (LECTURE SHEET) Straight Objective Type Questions (Boyle.s Law - applications)
5 Videos
- THERMAL PROPERTIES OF MATTERBOOK - AAKASH SERIESCHAPTER - THERMAL PROPERTIES OF MATTEREXERCISE - EXPANSION OF GASES (LECTURE SHEET) Straight Objective Type Questions (Kinetic theory of gases)
5 Videos
- THERMAL PROPERTIES OF MATTERBOOK - AAKASH SERIESCHAPTER - THERMAL PROPERTIES OF MATTEREXERCISE - EXPANSION OF LIQUIDS (PRACTICE SHEET) LEVEL-II (Integer/Subjective Type Questions)
4 Videos
- SYSTEM OF PARTICLES AND ROTATIONAL MOTIONBOOK - AAKASH SERIESCHAPTER - SYSTEM OF PARTICLES AND ROTATIONAL MOTIONEXERCISE - PRACTICE EXERCISE
99 Videos
- THERMODYNAMICSBOOK - AAKASH SERIESCHAPTER - THERMODYNAMICSEXERCISE - EXERCISE - 3
33 Videos
Similar Questions
An air bubble comes from the bottom to the surface of a lake of depth 2.5 m. The surface temperature of the lake is 40∘C. The diameter of the bubble at the bottom and at the surface are 3.6 mm and 4 mm respectively. Find the temperature of the lake at the bottom.
View SolutionTwo thermally insulated vessels 1 and 2 are filled with air at temperature (T1,T2), volume (V1,V2) and pressure (P1,P2) respectively. If the valve joining the two vessels is opened, the temperature inside the vessel at equilibrium will be
View SolutionIn figure shown, left arm of a U-tube is immersed in a hot water bath at temperature T, and right arm is immersed in a bath of melting ice, the height of monomeric liquid in respective columns is htandh0. Determine the coefficient of expansion of the liquid

View SolutionThe centre of mass of two particles with masses 2kg and 6 kg located at (1,1,1), (2,2,1) respectively has the coordinates
View SolutionA bullet of mass 10×10−3 moving with a speed of 20ms−1 hits an ice block (0∘C) of 990g kept at rest on a frictionless floor and gets embedded in it. If ice takes 50% of K.E, the amount of ice melted (in grams) approximately is (J=4.2J/cal) (latent heat of ice=80cal/g)
View SolutionPressure versus temperature graph of an ideal gas as shown in Fig. Corresponding density (rho) versus volume (V) graph will be
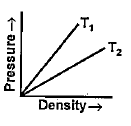
View SolutionFigure shows graphs of pressure versus density for an ideal gas at two temperatures T_(1) and T_(2) Which is correct ? .
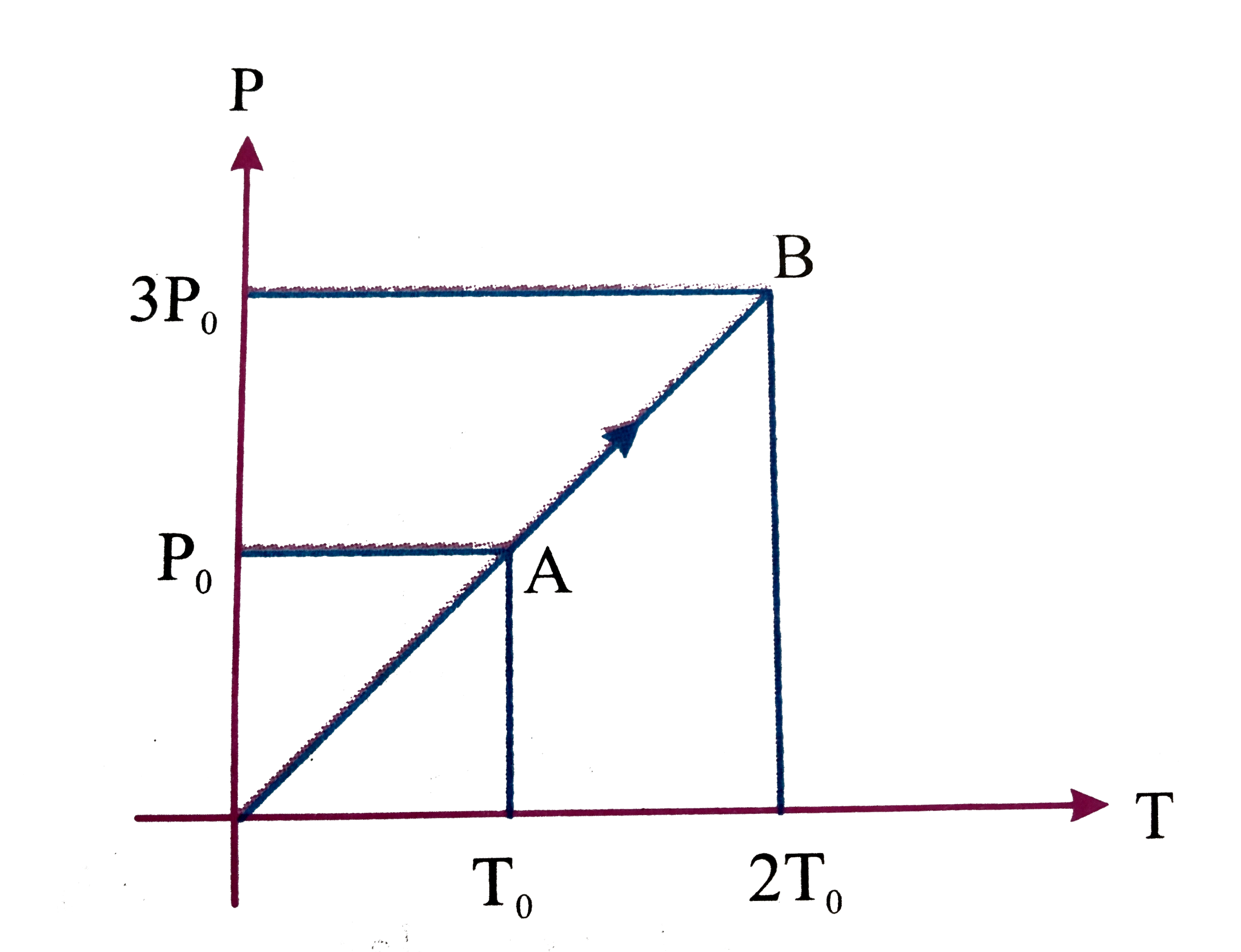
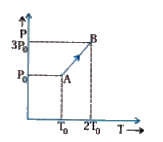
View SolutionPressure versus temperature graph of an ideal gas is shown in figure. Density of the gas at point A is P0. Density at B will be
View SolutionPressure versus density graph of an ideal gas is shown in figure
View SolutionFigure shows graphs of pressure versus density for an ideal gas at two temperatures T1 and T2 Then

View SolutionPressure versus temperature graph of an ideal gas is as shown in figure. Density of the gas at point A is ρ0 . Density at point B will be ____
View SolutionPressure versus density graph of an ideal gas is shown in figure
View SolutionPressure versus temperature graph of an ideal gas is shown in figure. Density of the gas at point A is rho_(0) . Density at B will be
View SolutionFigure shows graphs of pressure versus density for an ideal gas at two temperatures T_(1) and T_(2) Which is correct ? .
View SolutionFigure shows graphs of pressure versus density for an ideal gas at two temperatures T1 and T2. Which is correct:
View Solution

