Graph shows a hypothetical speed distribution. for a sample of N gas particles ( for v>v0,dNdV=0 )

The value of v0 is 2N
The ratio VavgV0=23
The ratio vrmsv0=1√2
Three fourth of the total particle has speed between 0.5v0andv0
The correct Answer is:A, B, C, D
Topper's Solved these Questions
- THERMAL PROPERTIES OF MATTERBOOK - AAKASH SERIESCHAPTER - THERMAL PROPERTIES OF MATTEREXERCISE - EXPANSION OF GASES (LECTURE SHEET) Linked Comprehension Type Questions
6 Videos
- THERMAL PROPERTIES OF MATTERBOOK - AAKASH SERIESCHAPTER - THERMAL PROPERTIES OF MATTEREXERCISE - EXPANSION OF GASES (LECTURE SHEET) Matrix Matching Type Questions
2 Videos
- THERMAL PROPERTIES OF MATTERBOOK - AAKASH SERIESCHAPTER - THERMAL PROPERTIES OF MATTEREXERCISE - EXPANSION OF GASES (LECTURE SHEET) Straight Objective Type Questions (Kinetic theory of gases)
5 Videos
- SYSTEM OF PARTICLES AND ROTATIONAL MOTIONBOOK - AAKASH SERIESCHAPTER - SYSTEM OF PARTICLES AND ROTATIONAL MOTIONEXERCISE - PRACTICE EXERCISE
99 Videos
- THERMODYNAMICSBOOK - AAKASH SERIESCHAPTER - THERMODYNAMICSEXERCISE - EXERCISE - 3
33 Videos
Similar Questions
Knowledge Check
Graph shows a hypothetical speed distribution for a sample of N gas particle (for V>V0,dNdV=0)
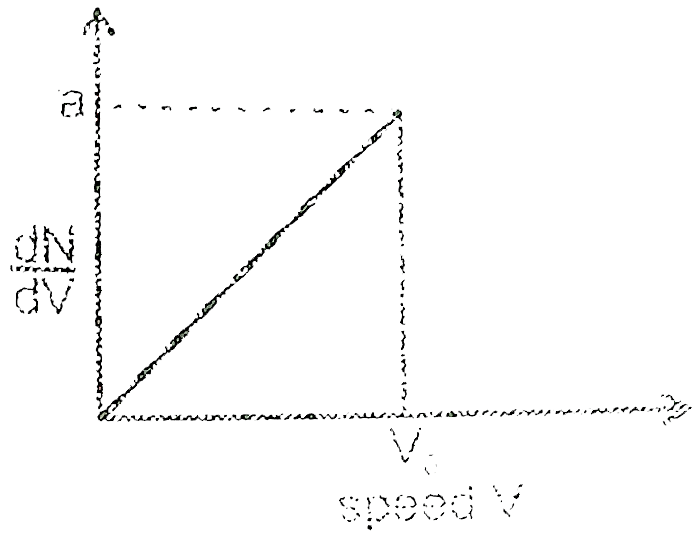 AThe value of V0 is 2NBThe ratio Vavg/V0 is equal to 2/3CThe ratio Vrms/V0 is equal to 1/√2DThree fourth of the total particle has a speed between 0.5V0 and V0.
AThe value of V0 is 2NBThe ratio Vavg/V0 is equal to 2/3CThe ratio Vrms/V0 is equal to 1/√2DThree fourth of the total particle has a speed between 0.5V0 and V0.Graph shows a hypothetical speed distribution for a sample of N gas particle :- (forV>V0,dNdV=0)
_E01_138_Q01.png) AThe value of aV0 is 2N.BThe ratio Vavg/V0 is equal to 2/3.CThe ratio Vavg/V0 is equal to 1/√2.DThree fourth of the total particle has a speed between 0.5V0 and V0.
AThe value of aV0 is 2N.BThe ratio Vavg/V0 is equal to 2/3.CThe ratio Vavg/V0 is equal to 1/√2.DThree fourth of the total particle has a speed between 0.5V0 and V0.For an adiabatic process graph between PV & V for a sample of ideal gas will be
A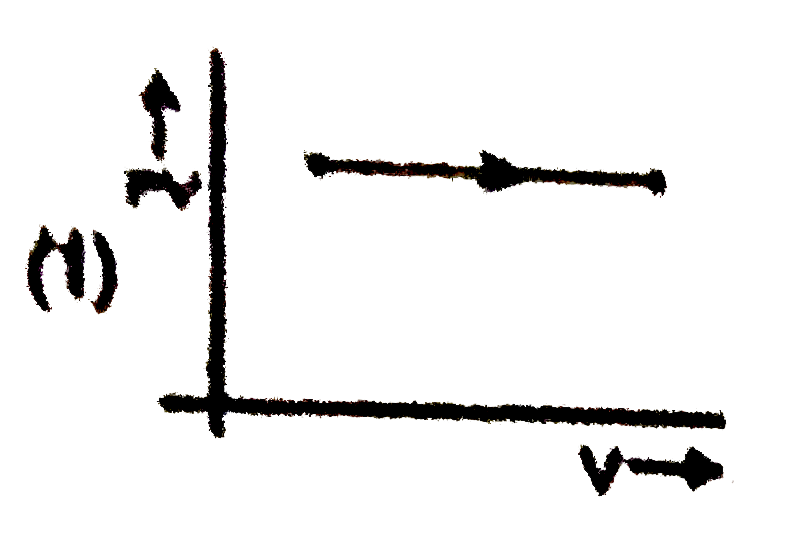 B
B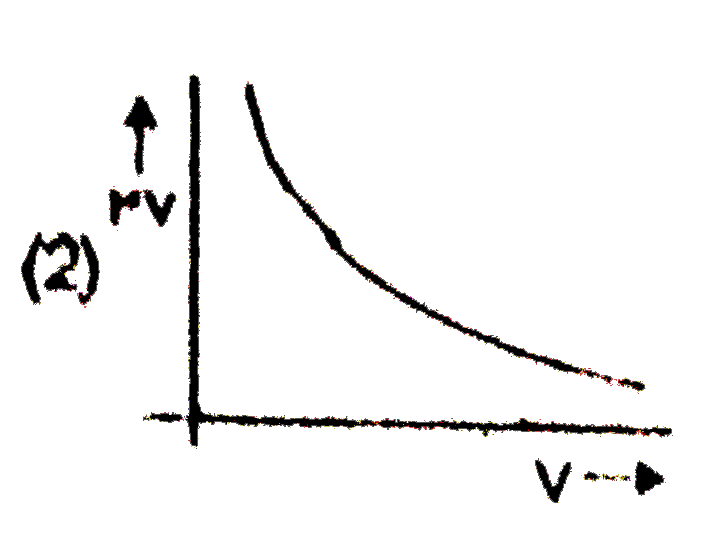 C
C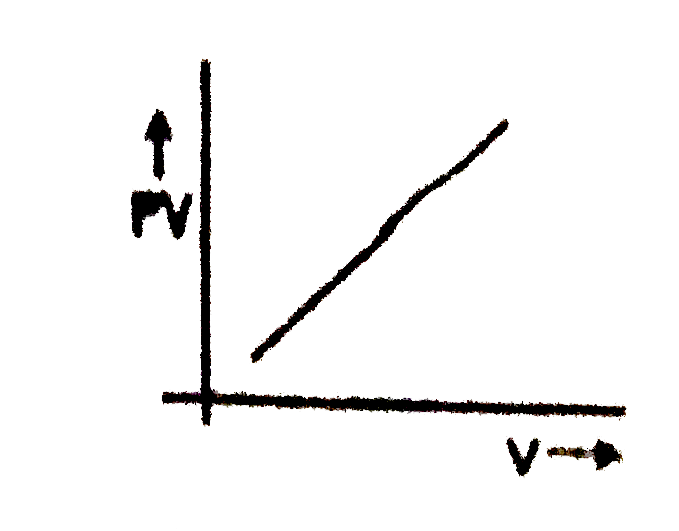 D
D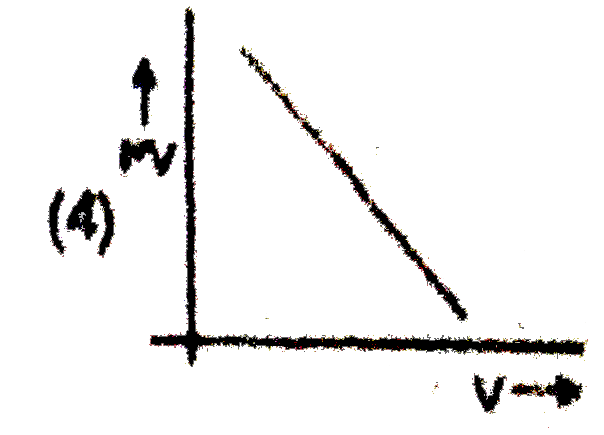
For an adiabatic process graph between PV & V for a sample of ideal gas will be
View SolutionWithin a spherical charge distribution of charge density ρ(r), N equipotential surface of potential V0,V0+ΔV,V0+2ΔV,…V0+NΔV(ΔV>0), are drawn and have increasing radii r0,r1,r2,….RN, respectively. If the difference in the radii of the surface is constant for all values of V0 and ΔV then :-
View SolutionWithin a spherical charge distribution of charge density ρ(r), N equipotential surface of potential V0,V0+ΔV,V0+2ΔV,…V0+NΔV(ΔV>0), are drawn and have increasing radii r0,r1,r2,….RN, respectively. If the difference in the radii of the surface is constant for all values of V0 and ΔV then :-
View SolutionWithin a spherical charge distribution of charge density ρ(r), N equipotential surface of potential V0,V0+ΔV,V0+2ΔV,…V0+NΔV(ΔV>0), are drawn and have increasing radii r0,r1,r2,….RN, respectively. If the difference in the radii of the surface is constant for all values of V0 and ΔV then :-
View SolutionThe root mean square speed of a group of gas moecules, having speeds v1,v2,....vN is
View Solution

