2NO2(g) (brown) ⇔N2O4(g),ΔH=57.2 kJ mol−1 if the reaction vessel kept in ice and hot water than what is the change in colour ?
This reaction is exothermic. So at lower temperature the forward reaction take place. So in mixture NO2 decreases so brown colour. decreases. If the vessel kept in hot water then the reaction take place in reverse direction and concentration of NO2 increases so brown colour increases.
Similar Questions
For the reaction 2NO2(g)⇔N2O4(g), when ΔS=−176.0JK−1mol−1andΔH=−57.8kJmol−1, the magnitude of ΔG at 298 K for the reaction is __________ kJ mol−1. (Nearest integer)
View SolutionFor the reaction 2NO2(g)⇔N2O4(g), when ΔS=−176.0JK−1andΔH=−57.8kJmol−1, the magnitude of ΔG at 298 K for the reaction is __________ kJ mol−1. (Nearest integer)
View Solution
Knowledge Check
The equilibrium system N2O4(g)⇔2NO2(g) has KP=11 and ΔH=57kJ.mol−1 at 25∘C. Which action will NOT cause a change in the position of the equilibrium?
AIncreasing the temperatureBAdding NO2(g)CAdding xenon gas to increase th pressureDIncrasing the container volumeFor the reaction N2(g)+3H2(g)⇔2NH3(g),ΔH=−93.6kJmol−1. The concentration of H2 at equilibrium will increase if
Athe temperature is loweredBthe volume of the system is decreasedCN2 is added at constant volumeDNH3 is addedThe enthalpy change (ΔH) for the process, N2H4(g)→2N(g)+4H(g) is
is 1724 kJ mol−1. If the bond energy of N-H bond in ammonia is 391 kJ mol−1, what is the bond energy for N-N bond in N2H4?A391 kJ mol−1B160 kJ mol−1C1173 kJ mol−1D320 kJ mol−1
For the reaction N2(g)+3H2(g)⇔2NH3(g),ΔH=−93.6kJmol−1. The concentration of H2 at equilibrium will increase if
View SolutionFor the following reaction at equilibrium
2NO2(g)↔N2O4(g) ΔH=−58KJ/mole
following change are made then identify in which direction reaction shift
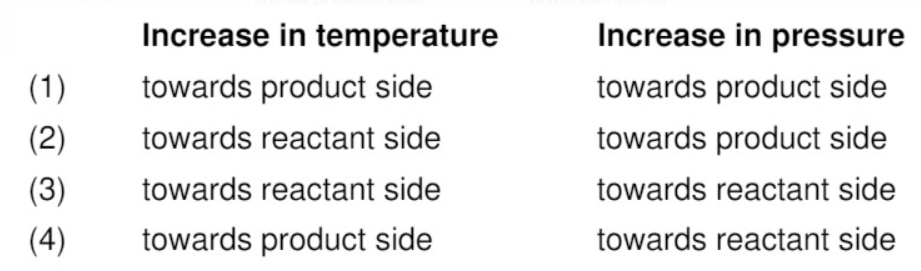
View SolutionFor the following reaction at equilibrium
2NO2(g)↔N2O4(g) ΔH=−58KJ/mole
following change are made then identify in which direction reaction shift

View SolutionThe enthalpy change (ΔH) for the process, N2H4(g)→2N(g)+4H(g) is
is 1724 kJ mol−1. If the bond energy of N-H bond in ammonia is 391 kJ mol−1, what is the bond energy for N-N bond in N2H4?
View SolutionThe enthalpy change (ΔH) for the process, N2H4(g)→2N(g)+4H(g) is
is 1724 kJ mol−1. If the bond energy of N-H bond in ammonia is 391 kJ mol−1, what is the bond energy for N-N bond in N2H4?
View Solution

