The correct order of acid strengths of benzoic acid (X), peroxybenzoic acid (Y) and p-nitrobenzoic acid (Z) is
A
Y>Z>X
B
Z>Y>X
C
Z>X>Y
D
Y>X>Z
Text Solution
The correct Answer is:C
The acidic strength depends upon the stabilisation of negative charge developed due to the donation of H+ ion.
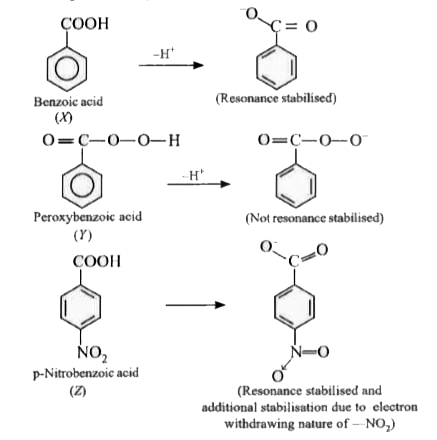
Hence, acidic strength order is Z>X>Y.
|
Similar Questions
Knowledge Check
The correct order of acid strength is
AHCIO4<HCIO3<HCIO2<HCIOBHCIO2<HCIO3<HCIO4<HCIOCHCIO4<HCIO<HCIO2<HCIO3DHCIO<HCIO2<HCIO3<HCIO4The correct order of acidic strength.
ACl2O7>SO2>P4O10BK2O>CaO>MgOCCO2>N2O5>SO3DNa2O>MgO>Al2O3Correct order of acidic strength
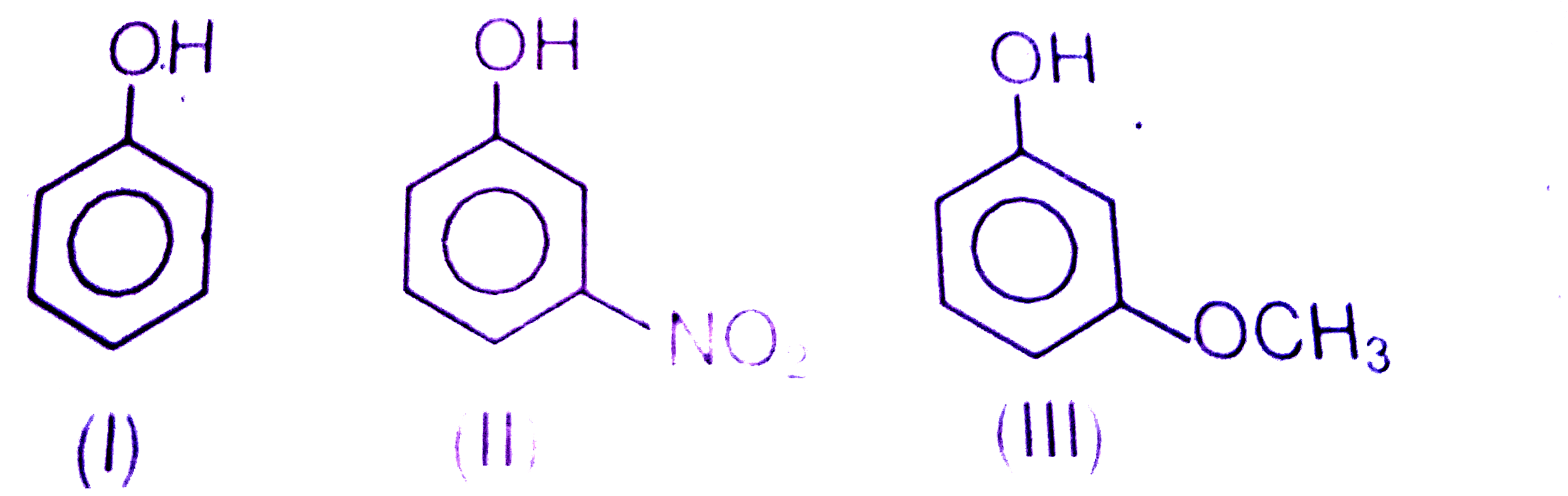 AI>II>IIIBII>III>ICI>III>IIDII>II>I
AI>II>IIIBII>III>ICI>III>IIDII>II>I
The correct order of acidic strength is
View SolutionThe correct order of acidic strength is
View SolutionThe correct order of acidic strength of carboxylic acid is
View SolutionThe correct order of acidic strength of carboxylic acid is
View SolutionThe correct increasing order of the acid strength of benzoic acid (I),4-nitrobenzoic acid (II),3,4-dinitrobenzoic acid (III) and 4-methoxybenzoic acid (IV) is
View Solution

