Depict the galvanic cell in which the reaction Zn(S)+Ag+(aq)→Zn2+(aq)+2Ag(S) takes place.
Further show:
(i) Which of the electrode is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
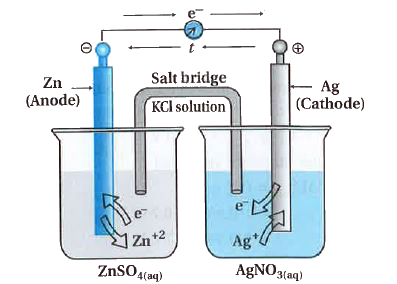
* The galvanic cell in which the given reaction takes place is depicted as:
Zn(S)∣∣Zn2+(aq)∣∣Ag+(aq)∣Ag(S)
(i) Zn electrode (anode) is negatively charged.
(ii) Ions are carriers of current in the cell and in the external circuit current will flow from silver to zinc.
(iii) The reaction taking place at the anode is
given by, Zn(S)→Zn2+(aq)+2e−
* The reaction taking place at the cathode is given by, Ag+(aq)+e−→Ag(S)
* On positive cathode of Ag, the Ag+ ions of solution will reduce by obtaining electron and particles of Ag will deposited on Ag electrode.
Ag+(aq)+e−→Ag(s) . . . . (Reduction)
EΘCr+∣Cr=−0.74V,EΘCd2+∣Cd=−0.40V
EΘAg+∣Ag=−0.80V,EΘFe3+∣Fe2+=0.77V
Topper's Solved these Questions
- ELECTROCHEMISTRYBOOK - KUMAR PRAKASHANCHAPTER - ELECTROCHEMISTRYEXERCISE - SECTION-D NCERT Exemplar Solution (MCQ.s)
17 Videos
- ELECTROCHEMISTRYBOOK - KUMAR PRAKASHANCHAPTER - ELECTROCHEMISTRYEXERCISE - SECTION-D NCERT Exemplar Solution (MCQ.s More Than One Options)
10 Videos
- ELECTROCHEMISTRYBOOK - KUMAR PRAKASHANCHAPTER - ELECTROCHEMISTRYEXERCISE - SECTION-B (Intext Questions and Answers)
14 Videos
- CHEMISTRY IN EVERYDAY LIFEBOOK - KUMAR PRAKASHANCHAPTER - CHEMISTRY IN EVERYDAY LIFEEXERCISE - SECTION - E (MCQs ASKED IN GUJCET/BOARD EXAM)
37 Videos
- GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSBOOK - KUMAR PRAKASHANCHAPTER - GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSEXERCISE - SECTION - E (MCQs ASKED IN GUJET/BOARD EXAMS)
46 Videos
Similar Questions
Which cell will measure standard electrode potential of copper electrode ?
View SolutionThe positive value of the standard electrode potential of Cu2+/Cu indicates that ____
View SolutionHow would you determine the standard electrode potential of the system Mg2+∣Mg ?
View SolutionTo prevent food from spoilage by micro organism, which substance is used ?
View SolutionDuring electrolysis of aqueous solution of Na2SO4 using graphite electrode .........
View SolutionDepict the galvanic cell in which the reaction Zn(s) +2Ag^(+)(aq) to Zn^(2+)(aq)+2Ag(s) takes place. Further indicate what are the carriers of current inside and outside the cell. State the reaction are each electrode.
View SolutionFormulate the galvanic cell in which the following reaction takes place: Zn(s)+2Ag^(+)(aq)toZn^(2+)(aq)+2Ag(s) State (i) Which one of the electrodes is negatively charged? (ii) The reaction taking place at each of its electrode. ltBrgt (iii) The carriers of current within this cell.
View SolutionDepict the galvanic cell in which the reaction Zn(s)+2Ag^(+)(aq)toZn^(2+)(aq)+2Ag(s) takes place. Further, show (i) which of the electrodes is negatively charged? (ii) the carriers of the current in the cell. (iii) Individual reaction at each electrode.
View Solutionउस गैल्वेनिक सेल को चित्रित कीजिए, जिसमें निम्न अभिक्रिया होती है ---
An(s)+2Ag+(aq)→Zn2+(aq)+2Ag(s)
अब बताइए कि--- कोन - सा इलेक्ट्रोड त्रण आवेशित है ?
View Solutionउस गैल्वेनिक सेल को चित्रित कीजिए, जिसमें निम्न अभिक्रिया होती है ---
An(s)+2Ag+(aq)→Zn2+(aq)+2Ag(s)
अब बताइए कि--- सेल में विद्युद्धारा का वाहक कोन है ?
View Solutionउस गैल्वेनिक सेल को चित्रित कीजिए, जिसमें निम्न अभिक्रिया होती है ---
An(s)+2Ag+(aq)→Zn2+(aq)+2Ag(s)
अब बताइए कि--- प्रत्येक इलेक्ट्रोड पर होने वाली अभिक्रियाएँ क्या है ?
View Solutionउस गैल्वेनीक सेल को दर्शाइए जिसमे निम्नलिखित अभिक्रिया होती है -
Zn(s)+2Ag+(aq)→Zn2+(aq)+2Ag(s), अब बताइए -
(i) कौन-सा इलेक्ट्रोड ऋणात्मक आवेशित है ?
(ii) सेल में विद्युत्-धारा के वाहक कौन-से हैं ?
(iii) प्रत्येक इलेक्ट्रोड पर होने वाली अभिक्रिया क्या है ?
View SolutionDepict the galvanic cell in which the reaction Zn(s)+2Ag^(+)(aq)rarr Zn^(2+)(aq)+2Ag(s) takes place. Further show : (iii) Individual reaction at each electrode.
View SolutionDepict the galvanic cell in which the reaction Zn(s) + 2Ag^(+) (aq) to Zn^(2+) (aq) + 2A(s) takes place. Further show. (i) Which of the electrode is negatively charged? (ii) The carriers of the current in the cell. (iii) Individual reaction at each electrode.
View SolutionDepict the galvanic cell in which the reaction Zn(s) +2Ag^(+)(aq)rarr Zn^(2+)(aq) + 2Ag(s) takes place . Further show : (i) which of the electrode is negatively charged, (ii) the carriers of the current in the cell, and (iii) individual reaction at each electrode.
View Solution

