Consider the following diagram in which an electrochemical cell is coupled to an electrolytic cell. What will be the polarity of electrodes A and B in the electrolytic cell ?
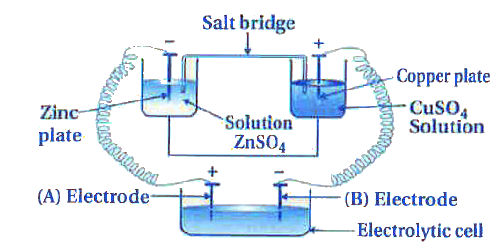
Text Solution
* Electrode (A) has negative electric charge and electrode (B) has positive electric charge, because electrochemical cell of Zn-Cu is attached with electrolytic cell.
* In electrochemical cell Zn is oxidized (Zn→Zn2++2e−) such electrons are moved on the Zn metal and hence, it is negative, so the electrode (A) attached with it become positive.
* In cell, copper ion undergoes reduction reaction and hence amount of electron get reduced and so copper become positive and the electrode attached with it become negative.
|
Similar Questions
Consider the following diagram in which an electrochemical cell is coupled to an electrolytic cell. What will be the polarity of electrodes 'A' an d 'B' in the electrolytic cell?
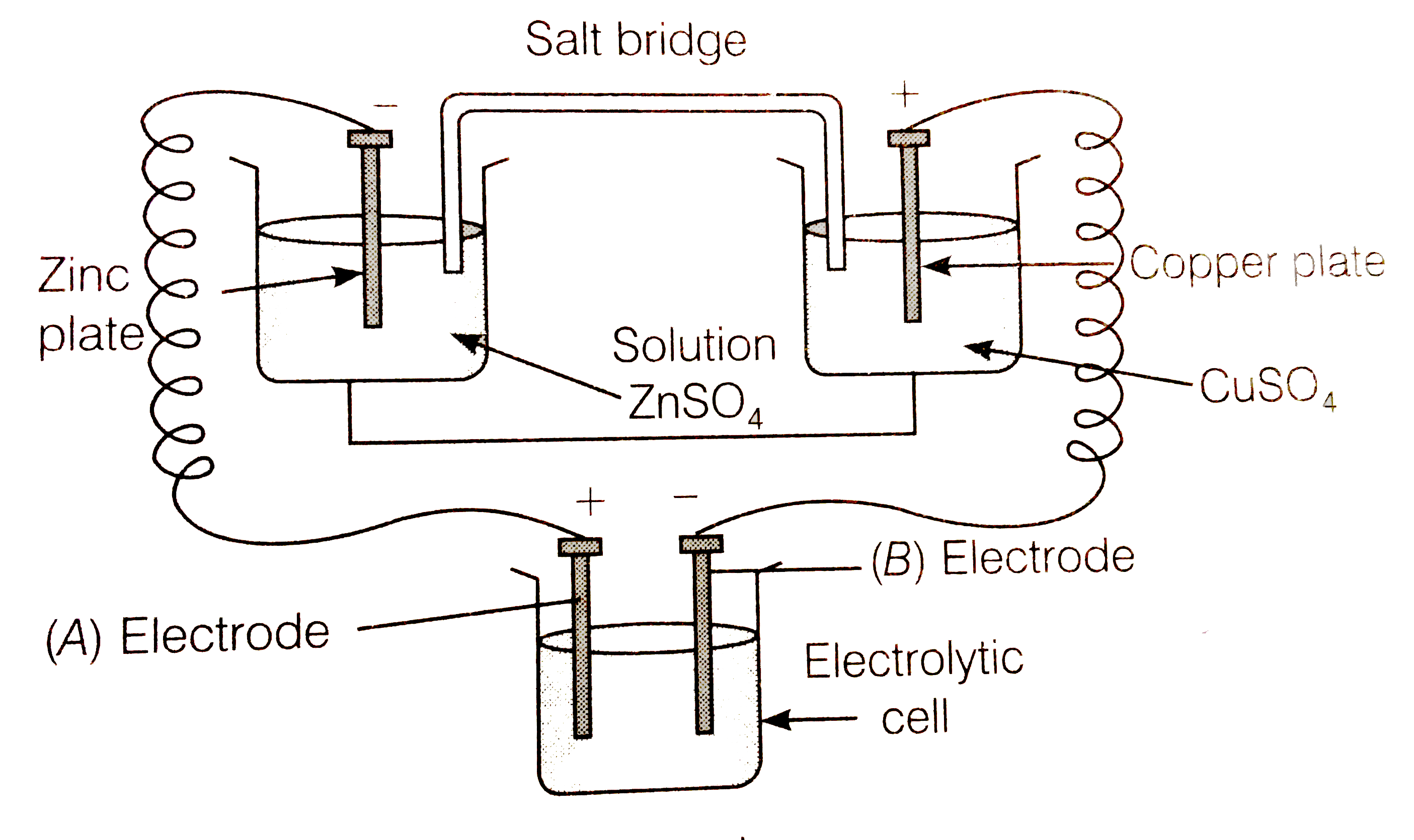
View SolutionConsider the following diagram in which an electrochemical cell is coupled to an electrolytic cell. What will be the polarity of electrodes 'A' an d 'B' in the electrolytic cell?

View Solution
Knowledge Check
An electrochemical cell an behave like an electrolytic cell when
AEcell=0BEcell>EextCEext>EcellDEcell=EextAn electrochemical cell an behave like an electrolytic cell when
AEcell=0BEcell>EextCEext>EcellDEcell=EextAn electrochemical cell can behave like an electrolytic cell when
AEcell=0BEcell>EextCEext>EcellDEcell=Eext
An electrochemical cell an behave like an electrolytic cell when
View SolutionElectrolytic cell
View SolutionWhat is an electrolytic cell?
View SolutionAn electrochemical cell an behave like an electrolytic cell when
View SolutionAn electrochemical cell an behave like an electrolytic cell when
View Solution

