Metal A,B and C has reduction potentials, 0.34 volt, -0.80 volt and -0.46 volt respectively. So give order of their capacity as reducing agent.
C>A>B
A>B>C
B>C>A
C>B>A
The correct Answer is:C
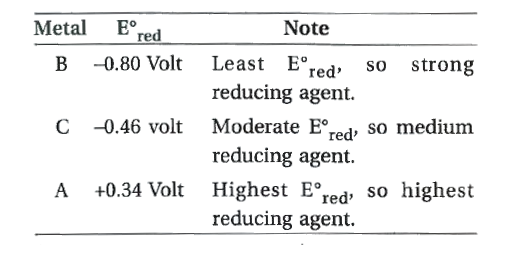
So, order of reducing agent is : B>C>A
Metal with less reduction potential (E∘red) will liberate electrons and easily get oxidized. Hence, it is strongest reducing agent.
Similar Questions
Consider the reaction 2Ag++Cd→2Ag+Cd2+. The standard reduction potentials of Ag+/AgandCd2+/Cd are + 0⋅80 volt and 0⋅40 volt, respectively.
Give the cell representation.
View SolutionConsider the reaction 2Ag++Cd→2Ag+Cd2+. The standard reduction potentials of Ag+/AgandCd2+/Cdare+0⋅80 volt and 0⋅40 volt, respectively.
What will be the emf of the cell if concentration of Cd2+is0⋅1MandAg+is0⋅2M?
View Solution
Knowledge Check
The value of E∘red for metals A,B and are 0.34 Volt , −0.80 Volt and −0.46 volt respectively. State the CORRECT order for their ability toact as reducing agent.
AC>B>ABA>B>CCB>C>ADC>A>BFour metals A, B, C and D are having standard reduction potential as -3.06, -1.66, -0.40 and 0.80 volt respectively. The most reactive metal is
ADBACCDBThe standard electrode potentials of Zn, Ag and Cu are -0.76, 0.80 and 0.34 volt respectively, then:
AAg can oxidised Zn and CuBAg can reduce Zn2+ and Cu2+CZn can reduce Ag+ and Cu2+DCu can oxidise Zn and Ag
Reduction potentials of 4 metals A, B, C and D are −1.66V,+0.34V,+0.80Vand−0.76V. What is the order of their reducing power and reactivity ?
View SolutionThe standard reduction potential of normal calomel electrode and reduction potential of saturated calomel electrodes are 0.27 and 0.33 volt respectively. What is the concentration of Cl− in saturated solution of KCl?
(a)0.1M
(b)0.01M
(c)0.001M
(d)None
View SolutionConsider the reaction 2Ag++Cd→2Ag+Cd2+. The standard reduction potentials of Ag+/AgandCd2+/Cdare+0⋅80 volt and 0⋅40 volt, respectively.
What is the standard cell emf, E∘ ?
View SolutionConsider the reaction 2Ag++Cd→2Ag+Cd2+. The standard reduction potentials of Ag+/AgandCd2+/Cdare+0⋅80 volt and 0⋅40 volt, respectively.
Will the cell work spontaneously for the condition given in (iii) above?
View SolutionThe standard reduction potential of normal calomel electrode and reduction potential of saturated calomel electrodes are 0.27 and 0.33 volt respectively. What is the concentration of Cl− in saturated solution of KCL?
View Solution

