The number of d-electrons in Fe2=(Z=26) is not equal to the number p of electrons in which one of the following?
A
p-electrons inCl (Z= 17)
B
d - electrons in Fe (Z = 26)
C
p- electrons in Ne (Z = 10)
D
s - electrons in Mtg (Z = 12)
Text Solution
The correct Answer is:a
p-electrons inCl (Z= 17)
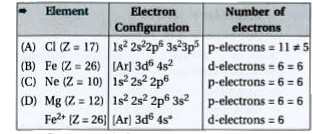
Fe2+ has 6 d-electrons.
(Option - A) has 11 p - el electrons , which are not equal to six d- electrons in Fe2−
|
Similar Questions
Knowledge Check
The number of d-electrons in Fe2+(Z=26) is not equal to the number of electrons in which one of the following ?
Ad-electrons in Fe (Z = 26)Bp-electrons in Ne (Z = 10)Cs-electrons in Mg (Z = 12)Dp-electrons in Cl (Z = 17)The number of d-electrons in Fe2+(Z=26) is not equal to the number of electrons in which one of the following ?
Ad- electrons in Fe (Z= 26)Bp- electrons in Ne (Z = 10 )Cs-electrons in Mg (Z= 12 )Dp -electrons in C1 (Z =17)The number of d-electrons in Fe2+(Z=26) is not equal to the number of electrons in which one of the following ?
As-electrons in Mg (Z=12)Bp-electrons in Cl (Z=17)Cd-electrons in Fe (Z=26)Dp-electrons in Ne (Z=10)
The number of d-electrons in Fe2+(Z=26) is not equal to the number of electrons in which one of the following ?
View SolutionThe number of d-electron in Fe2+(Z=26) is not equal to the number of electron in which one of the following?
View SolutionThe number of d-electrons in Fe2+(Z=26) is not equal to that of :
View SolutionTne number of 'd' electron is Fe+2(Z=26) is equal to that of the
View SolutionNumber of unpaired electrons in Fe3+ (Z = 26) is
View Solution

