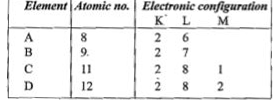
Which element is most electropositive and most electronegative respectively ?
A
K, Cl
B
Na. I
C
Cs, F
D
Na. F
Text Solution
The correct Answer is:c

Na, K, Cs are alkali metals of group one. Among the three Cs has least electronegativity and is most electropositive.
Cl and F are halogen element of group seven. Among the two F is more electronegative and F is most electronegative, among all.
|
Similar Questions
Knowledge Check
Which halogen is most electropositive ?
AFBClCBrDIWhich element is the most electronegative?
AA(2,2)BB(2,3)CC(2,4)DD(2,5)Most electronegative element is
ANBOCFDCl
The most electronegative element is
View SolutionThe most electronegative element is
View SolutionThe most electronegative element is
View SolutionStatement-I : Cs and F combines violently to form CsF.
Statement-II : Cs is most electropositive and F is most electronegative.
View SolutionWhich of the following is most electropositive
View Solution