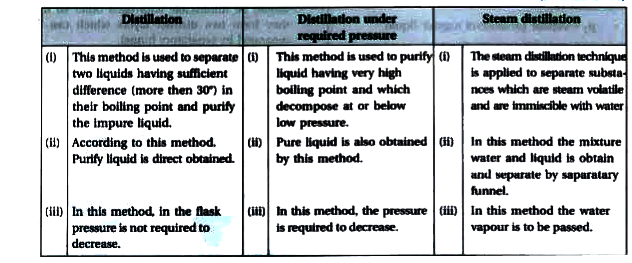
What is the difference between distillation, distillation under reduced pressure and steam distillation?
Text Solution
|
Similar Questions
Knowledge Check
Distillation under reduced pressure is employed for
AC6H6BPetrolCCH2OHCHOHCH2OHDOrganic compounds used in medicine.Distillation under reduced pressure is used to purify liquids which:
Aare highly volatileBhave high boiling pointsCare explosivesDdecomposes below their boiling pointsSteam distillation is based on
ABoyle's lawBCharle's lawCDalton's law of partial pressuresDAvogadro's law
Why is impure glycerol by distillation under reduced pressure ?
View SolutionPartition Chromatography|Principle|Fractional Distillation|Procedure|Distillation Under Reduced Pressure|Steam Distillation|Differential Extraction|Summary
View SolutionSteam distillation is based on
View SolutionAssertion: Glycerol is purified by distillation under reduced pressure.
Reason: Method of distillation under reduced pressure is used to purify liquids having very high boiling points and those, which decompose at or below their boiling points.
View SolutionAssertion: Glycerol is purified by distillation under reduced pressure.
Reason: Method of distillation under reduced pressure is used to purify liquids having very high boiling points and those, which decompose at or below their boiling points.
View Solution