Which of the following compounds contain after the carbon atoms in the same hybridisation state?
H−C≡C−C≡C−H
CH3−C≡C−CH3
CH2=C=CH2
CH2=CH−CH=CH2
The correct Answer is:A, D
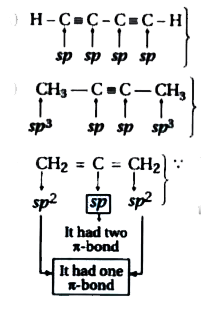
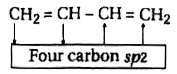 ∵ Every carbon have one double bond
∵ Every carbon have one double bond
Topper's Solved these Questions
- ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESBook:KUMAR PRAKASHANChapter:ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESExercise:Section -D -Short Answer Type
Explore 54 Videos
- ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESBook:KUMAR PRAKASHANChapter:ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESExercise:Section -D - Matching the Columns
Explore 10 Videos
- ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESBook:KUMAR PRAKASHANChapter:ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESExercise:Section -D (Multiple Choice Questions MCQs)
Explore 30 Videos
- HYDROGENBook:KUMAR PRAKASHANChapter:HYDROGENExercise:Section-D NCERT Exemplar (Long Answer)
Explore 8 Videos
- QUESTION ASKED IN JEE -2018Book:KUMAR PRAKASHANChapter:QUESTION ASKED IN JEE -2018Exercise:MCQ
Explore 10 Videos
Similar Questions
(I) CH3−CH2−CH2−CH2−OH
(II) CH3−CH2−CH∣OH−CH3
(III) CH3−CH3∣C∣OH−CH3
(IV) CH3−CH∣CH3−CH2−OH
(V ) CH3−CH2−O−CH2−CH3 (VI) CH3−O−CH2−CH2−CH3 (VII) CH3−O−CH∣CH3−CH3
Which of the above compounds form pairs of metamers?
View SolutionMatch the type of mixture of compounds in Column-I with the technique of separation/purification given in Column-II

View SolutionWhich of the following is the correct IUPAC name ?
View SolutionAtomic hydrogen combines with almost all elements but molecular hydrogen does not. Explain.
View SolutionAccording to molecular orbital theory, which of the following will not be a viable molecule?
View SolutionElectronegativity of carbon atoms depends upon their state of hybridisation in which of the following compounds the carbon marked asterisk, is most electronegative ?
View SolutionWhich of the following compounds contain all the carbon atoms in the same hybrisation state ?
View SolutionWhich of the following compounds contain all the carbon atoms in the same hybridisation state ?
View SolutionElectronegativity of carbon atoms depends upon their state of hybridisation. In which of the following compounds, the carbon marked with asterisk is most electronegative ?
View SolutionWhich of the following compounds contain all the carbon atoms in the same hybridisation state ?
View SolutionElectronegativity of carbon atoms depends upon their state of hybridisation. In which of the following compounds, the carbon marked with asterisk is most electronegative?
View SolutionElectronegativity of carbon atoms depends upon their state of hybridisation. In which of the following compounds, The carbon marked with asterisk is most electronegative ?
View SolutionIn which of the following all carbon atoms are of same hybridisation
View SolutionWhich of the following compounds have the same type of hybridised carbon atoms?
View SolutionIn which of the following all carbon atoms are of same hybridisation
View Solution

