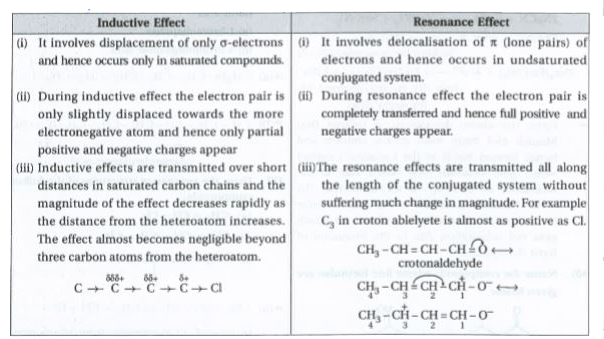
Give three points of difference between inductive effect and resonance effect
Text Solution
|
Similar Questions
Knowledge Check
The inductive effect
Aimplies atom's ability to cause bond polarizationBimplies transfer of lone pair of electrons from lesser electronegative atom to the more electronegative atom in a moleculeCimplies transfer of lone pair of electrons from more electronegative atom to the lesser electronegative atom in a moleculeDincreases with increase of distance.The inductive effect
Adecreases with increase of distanceBits extent increases with increase of distanceCindicates the transfer of π pair of electrons from less electronegative atom to more electronegative atom in a moleculeDshows the transfer of lone pair of electrons.In which of the following molecule/ions are the inductive effect, resonance effect, and hyperconjugation all operating simultaneously?
A(CH3)+CB(CH3)3C−+CH2CCH2=CH−CH=ODCH3CH=CH−CH=O
What are the main points of difference between inductive and electromeric effects ?
View Solution|| Inductive effect ||
View SolutionDifferentiate between inductive and electromeric effect
View SolutionState any two differences between Peltier effect and Joule effect.
View SolutionCompare inductive effect with mesomeric effect.
View Solution