The hydrolysis of optically active 2- bromobutane with aqueous NaOH result in the formation of Solution in Kannada
A
(±) butan - 2- ol
B
(±) butan - 1- ol
C
(-) butan - 2- ol
D
(+) butan - 2- ol
Video Solution
Text Solution
The correct Answer is:A
Usually 1∘ carbocation followes second order kinetics and 2∘and3∘ carbocation follows first order kinetics.
The reaction between 2- tromobutonewith equal NaOH follows first order kinetic, i.e.,the rate of reaction depends upon the concentration of 2-brombutane (SN1 mechanism).
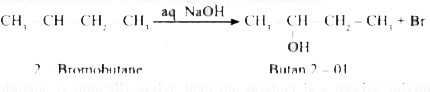
Since, the 2-bromobutane is optically active than the produt is a receive mixture. This is because carbocations are as intermadiate in SN1 reactions. Since, carbonation being Sp2-hybridized is planar species, the attack of the nucleotide on it can occur from both the face with almost case giving a 50 : 50 mixture of two enantiomes.
|
Similar Questions
Explain the hydrolysis of 2-bromobutane with aqueous KOH.
View SolutionElimination of bromine from 2-bromobutane results in the formation of
View Solutionजलीय NaOH के साथ प्रकाशीय सक्रीय ब्रोमोब्यूटेन का जलअपघटन किसके निर्माण का परिणाम है
View SolutionDraw the structure of the optically active isomers of 2-bromobutane.
View Solution2-bromobutane is optically active but 1-bromobutane is optically inactive
View SolutionElimination of HBr from 2 bromobutane results in the formation of .
View SolutionElimination of bromine from 2-bromobutane results in the formation of:
View Solution
