Which of the following given statesment is incorrect?
F-centres generation is responsible factor for imparting colour to the crystal
Frenkel defect is usually shown by ionic compound having low coordination number
Stoichiometry of crystal remains uneffected due to schottky defect
Density of crystal always increses due to susbtitutional impurity defect
The correct Answer is:D
To determine which of the given statements is incorrect, we need to analyze each statement one by one based on our knowledge of crystal defects and their effects.
1. Analyze the First Statement:
- Statement: "App-Centered generation is responsible factor for impacting color to the crystal."
- Analysis: This statement is correct. App-Centered generation does lead to the excitation and de-excitation of electrons, which can affect the color of the crystal.
2. Analyze the Second Statement:
- Statement: "Frankl defect is usually shown by ionic compound having low coordination number."
- Analysis: This statement is also correct. The Frankl defect occurs when a smaller cation leaves its original position and occupies an interstitial site, which can lead to a reduction in coordination number.
3. Analyze the Third Statement:
- Statement: "Strychometry of a crystal remain unaffected due to Scott's defect."
- Analysis: This statement is correct. In Scott's defect, equal numbers of cations and anions are missing, which means the stoichiometry of the crystal remains constant.
4. Analyze the Fourth Statement:
- Statement: "Density of a crystal always increases due to substitutional impurity defect."
- Analysis: This statement is incorrect. The density of a crystal can either increase or decrease due to substitutional impurities depending on the size and mass of the substituting atoms compared to the original atoms. If the substituting atom is lighter or smaller, the density may decrease.
Conclusion:
The incorrect statement is: "Density of a crystal always increases due to substitutional impurity defect."
---
- Statement: "App-Centered generation is responsible factor for impacting color to the crystal."
- Analysis: This statement is correct. App-Centered generation does lead to the excitation and de-excitation of electrons, which can affect the color of the crystal.
- Statement: "Frankl defect is usually shown by ionic compound having low coordination number."
- Analysis: This statement is also correct. The Frankl defect occurs when a smaller cation leaves its original position and occupies an interstitial site, which can lead to a reduction in coordination number.
- Statement: "Strychometry of a crystal remain unaffected due to Scott's defect."
- Analysis: This statement is correct. In Scott's defect, equal numbers of cations and anions are missing, which means the stoichiometry of the crystal remains constant.
- Statement: "Density of a crystal always increases due to substitutional impurity defect."
- Analysis: This statement is incorrect. The density of a crystal can either increase or decrease due to substitutional impurities depending on the size and mass of the substituting atoms compared to the original atoms. If the substituting atom is lighter or smaller, the density may decrease.
The incorrect statement is: "Density of a crystal always increases due to substitutional impurity defect."
Similar Questions
Knowledge Check
In the reaction given below :
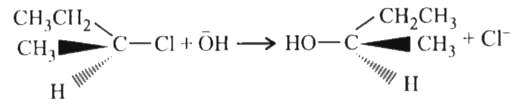
Which of the following statements is/are incorrect ?AThe reaction proceeds via SN2 mechanism hence inversion of configuration takes place.BThe reaction proceeds via SN1 mechanism hence inversion of configuration takes place.CThe reaction proceeds via SN2 mechanism hence their is no change in the configuration.DThe reaction proceeds via SN1 mechanism hence there is no change in the configuration.Which of the following statement is incorrect ? .
AJoule-Thomson coefficient is zero at inversion temperature of a real gas .BIdeal gas do not show Joule-Thomson effectCInversion temperature of H2 and he is very-very lowDJoule Thomson coefficient μ=(δPδT)H .Which of the following statement is incorrect ? .
Agas molecules are always evenly disturbed in atmosphereBvibrational motion is present in mono-atomic gasesCthe molecules in a gas are in rapid random motionDnone of these
Which of the following statement is incorrect for the given figure?
View SolutionWhich of the following statement is incorrect about the given energy profile diagram.
_2019_CBT4_E01_018_Q01.png)
View Solution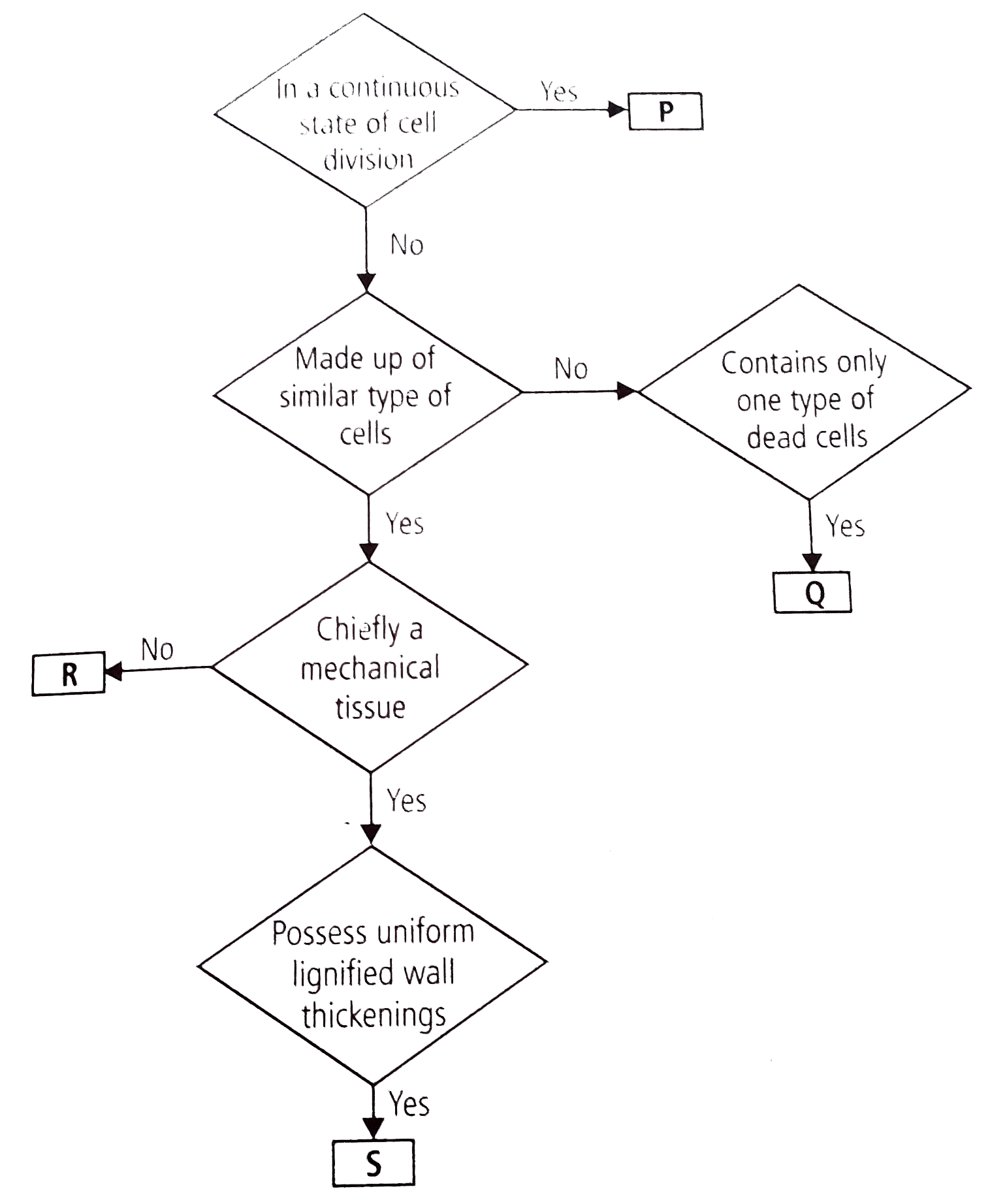
Study the flow chart given below:
Which of the following statements is incorrect regarding this?
View SolutionStudy the picture given below

Which of the following statement is incorrect about given picture ?
View SolutionWhich of the following statements is incorrect
View Solution