Positive and negative charge in 18 cc of water is
q=(±)9.63X105C
q=(±)1.63X105C
q=(±)9.63X10−5C
q=(±)2.63X10−5C
The correct Answer is:A
To find the positive and negative charge in 18 cc of water, we can follow these steps:
Step 1: Determine the mass of water
Given that the density of water is 1 gram per cubic centimeter (g/cm³), we can calculate the mass of 18 cc of water.
Mass=Density×Volume=1g/cm3×18cm3=18grams
Step 2: Calculate the number of water molecules
The molecular formula of water is H₂O, which means each molecule contains 2 hydrogen atoms and 1 oxygen atom. The molar mass of water is approximately 18 g/mol.
Using Avogadro's number (approximately 6.02×1023 molecules/mol), we can find the number of molecules in 18 grams of water:
Number of moles=MassMolar mass=18g18g/mol=1mol
Number of molecules=1mol×6.02×1023molecules/mol=6.02×1023molecules
Step 3: Calculate the number of electrons
Each water molecule (H₂O) has 10 electrons (2 from hydrogen and 8 from oxygen). Therefore, the total number of electrons in 18 cc of water is:
Total electrons=10electrons/molecule×6.02×1023molecules=6.02×1024electrons
Step 4: Calculate the total charge
The charge of a single electron is approximately −1.6×10−19 coulombs. Thus, the total negative charge due to electrons is:
Total negative charge=Number of electrons×Charge of one electron
=6.02×1024×(−1.6×10−19)=−9.632×105coulombs
Since protons have the same magnitude of charge but are positive, the total positive charge is:
Total positive charge=9.632×105coulombs
Final Answer
The positive and negative charge in 18 cc of water is approximately:
±9.63×105coulombs
Conclusion
Thus, the correct option is:
q = ± 9.63 × 10⁵ coulombs.
---
Given that the density of water is 1 gram per cubic centimeter (g/cm³), we can calculate the mass of 18 cc of water.
The molecular formula of water is H₂O, which means each molecule contains 2 hydrogen atoms and 1 oxygen atom. The molar mass of water is approximately 18 g/mol.
Each water molecule (H₂O) has 10 electrons (2 from hydrogen and 8 from oxygen). Therefore, the total number of electrons in 18 cc of water is:
The charge of a single electron is approximately −1.6×10−19 coulombs. Thus, the total negative charge due to electrons is:
The positive and negative charge in 18 cc of water is approximately:
Thus, the correct option is:
q = ± 9.63 × 10⁵ coulombs.
Topper's Solved these Questions
Similar Questions
Knowledge Check
Positive and negative charges of equal magnitude lie along the symmetry axis of a cylinder. The distance from the positive charge to the left - end cap of the cylinder is the same as the distance from the negative charge to the right - end cap.
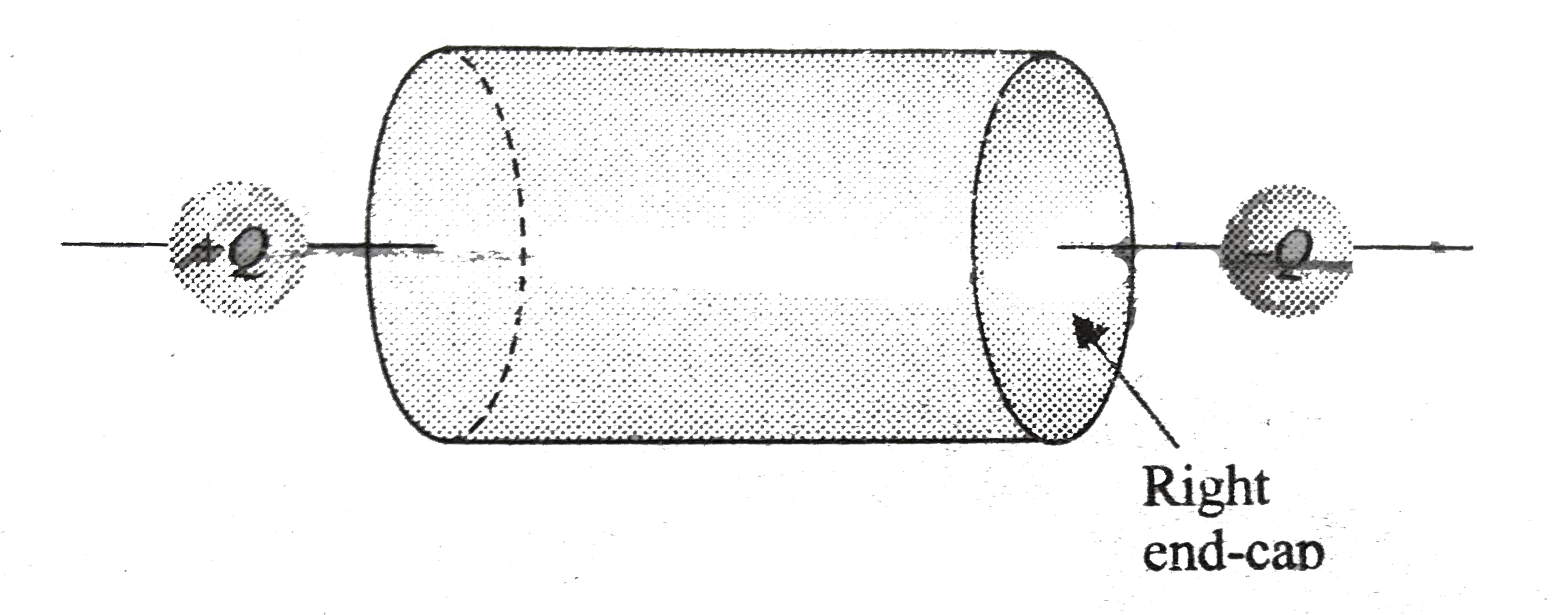
What is the flux of the electric field through the closed cylinder ?AzeroB+Q/ε0C+2Q/ε0D−Q/ε0A water molecule has an electric dipole moment C-m when it is in vapour state .The distance between center of positive and negative charge of the molecules
A5×10−11mB2×10−11mC4.1×10−3mD4.375×10−11m
Find the total negative and positive charges in 500 cc of water Given: Avogadro's number is 6.023×1023g−1mol−1, molecular weight of water in 18.
View SolutionHow much positive and negative charge is there in a cup of water ?
View SolutionPositive and negative charges of equal magnitude lie along the symmetry axis of a cylinder. The distance from the positive charge to the left - end cap of the cylinder is the same as the distance from the negative charge to the right - end cap.

What is the sign of the flux through the right - end cap of the cylinder ?
View SolutionFig (a) and (b) show the field lines of a single positive and negative charges respectively .
Does the kinetic energy of a small negative charge increase or decrease in going from B to A ?

View SolutionHow are positive and negative charges produced during a thunderstorm?
View SolutionWhat are positive and negative charges?how are these produced?
View Solution
