Assertion (A) : Carboxylic acids show acidic properties.
Reason (R) : The carboxylate ion after the removal of proton from carboxylic acids gets stabilised by resonance.
A
Both Assertion (A) and Reason (R) are correct statements, and Reason (R) is the correct explanation of the Assertion (A).
B
Both Assertion (A) and Reason (R) are correct statements, but Reason (R) is not the correct explanation of the Assertion (A).
C
Assertion (A) is correct, but Reason (R) is incorrect statement.
D
Assertion (A) is incorrect, but Reason (R) is correct statement
Text Solution
The correct Answer is:A
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
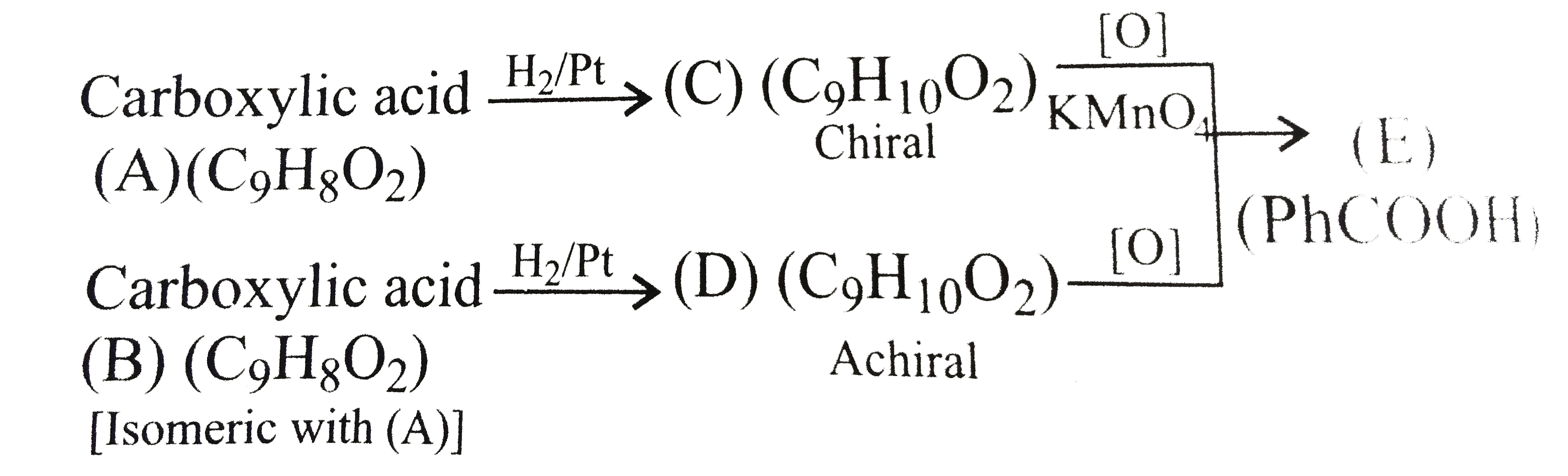
Carboxylic acid (A) is:A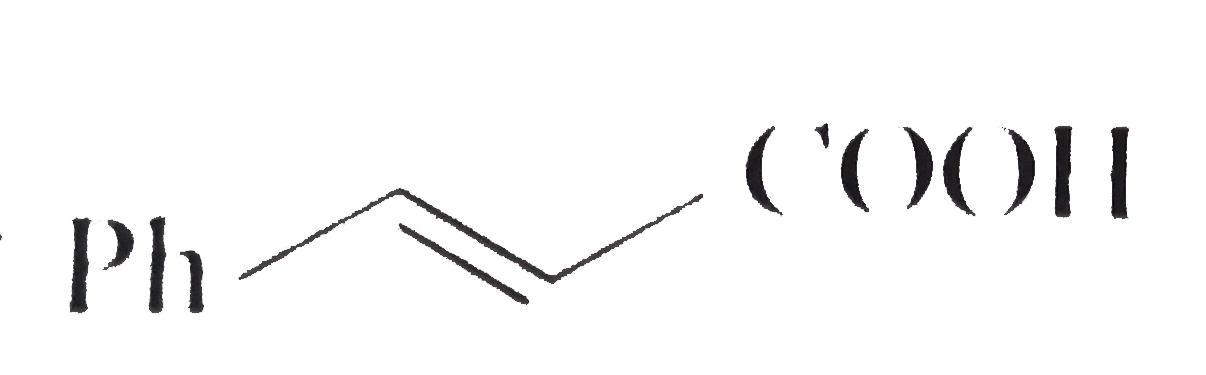 B
B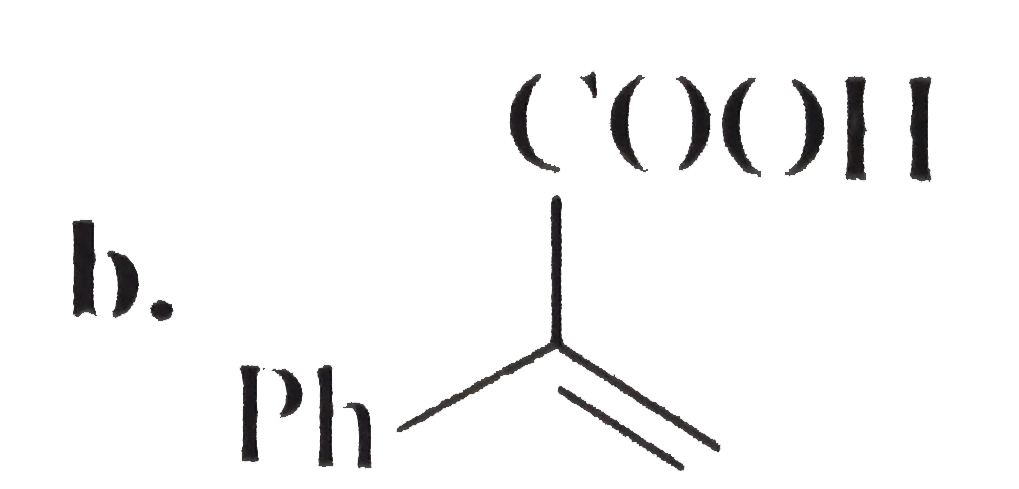 C
C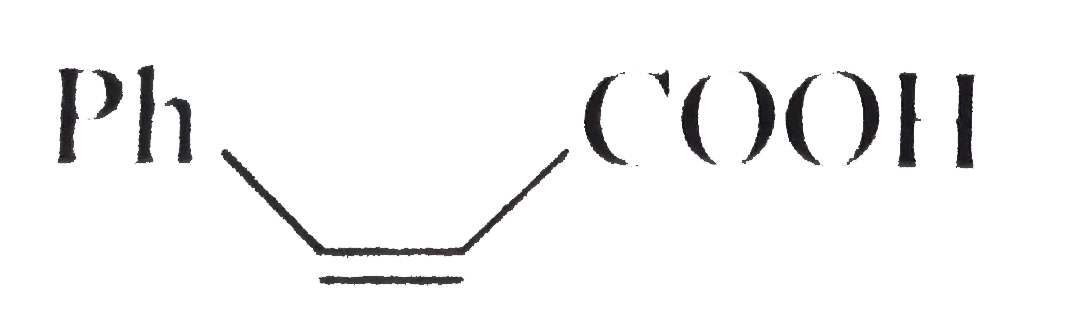 DBoth (a) and (c )
DBoth (a) and (c )Carboxylic acids are acidic in nature because :
Ait dissociates to give H+ ioinsBit dissolves in water to form protonsCthe carboxylate ion is resonance unstablisedDboth (a) and (b)Consider the acidity of the following carboxylic acids
APhCOOHBa−NO2C6H4COOHCp−NO2C6H4COOHDm−NO2C6H4COOH
Chemical properties of Carboxylic acids
View SolutionQuestions based on chemical properties of Carboxylic acids
View SolutionAssertion: Carboxylic acid exist as dimer.
Reason: Carboxylic acid shows hydrogen bonding.
View SolutionCarboxylic acid donot give characteristic property of
View SolutionCarboxylic acids do not give the characteristic properties of :
View Solution

