Why are carboxylic acids more acidic than alcohols or phenols although all of them have hydrogen linked to a oxygen atom?
Text Solution
The anions left after is removal of hydrogen from carboxy acid, alcohol and phenol are RCOO−, Ro− and ArO− respectively.
Out ofthese RCOO− is stabilised to the maximum extent. Therefore carboxy acids are more acidic than alcohol or phenols.
|
Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:U-LIKE SERIESChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:LONG ANSWER QUESTIONS - I
Explore 29 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:U-LIKE SERIESChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:LONG ANSWER QUESTIONS - II
Explore 44 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:U-LIKE SERIESChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:VERY SHORT ANSWER QUESTIONS
Explore 53 Videos
- ALCOHOLS, PHENOLS AND ETHERSBook:U-LIKE SERIESChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:SELF ASSESSMENT TEST (SECTION A)(MULTIPLE CHOICE QUESTIONS)(CHOOSE THE CORRECT OPTION)
Explore 6 Videos
- AMINESBook:U-LIKE SERIESChapter:AMINESExercise:SELF ASSESSMENT TEST
Explore 6 Videos
Similar Questions
Knowledge Check
Phenol is more acidic than
A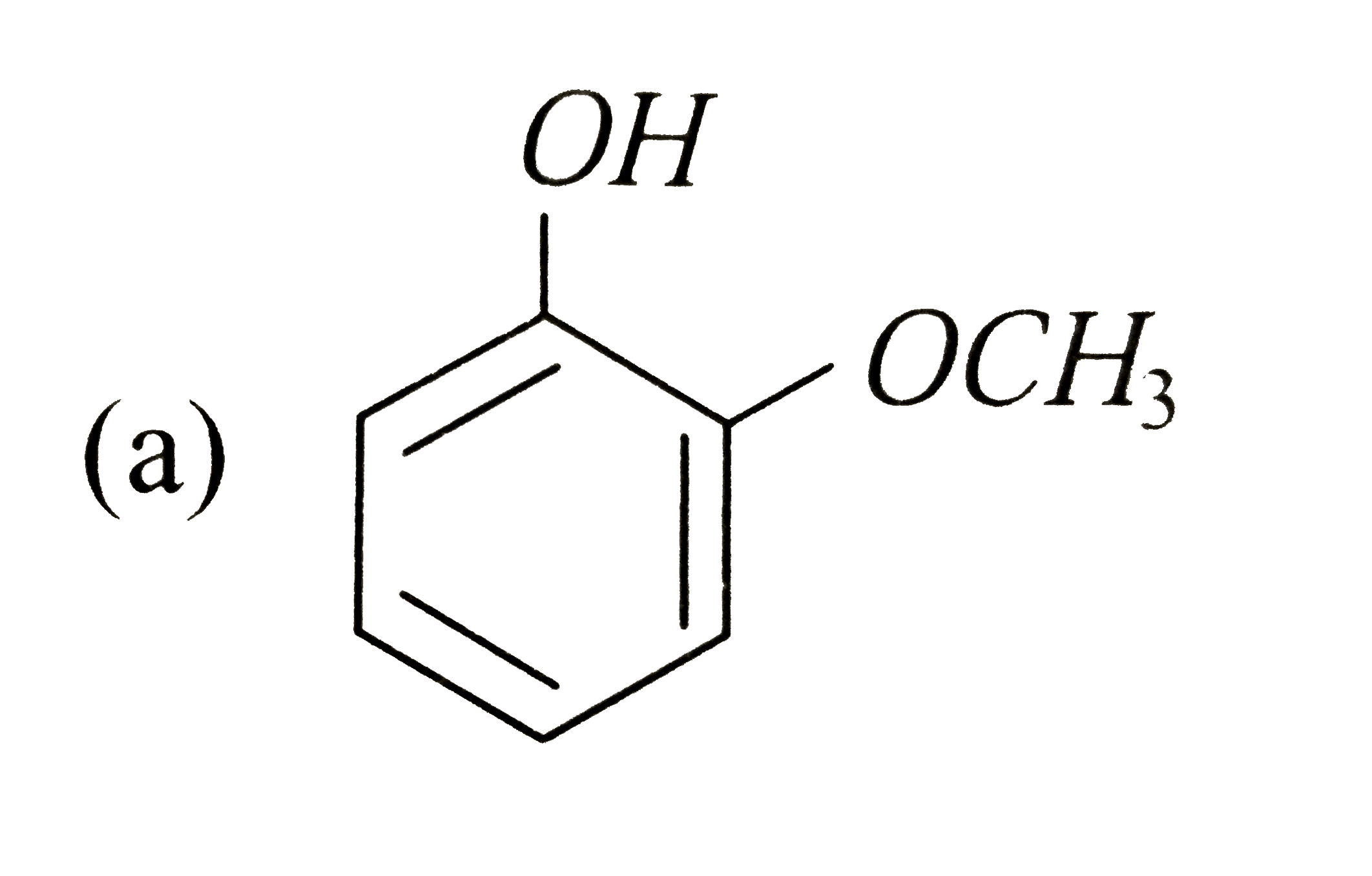 B
B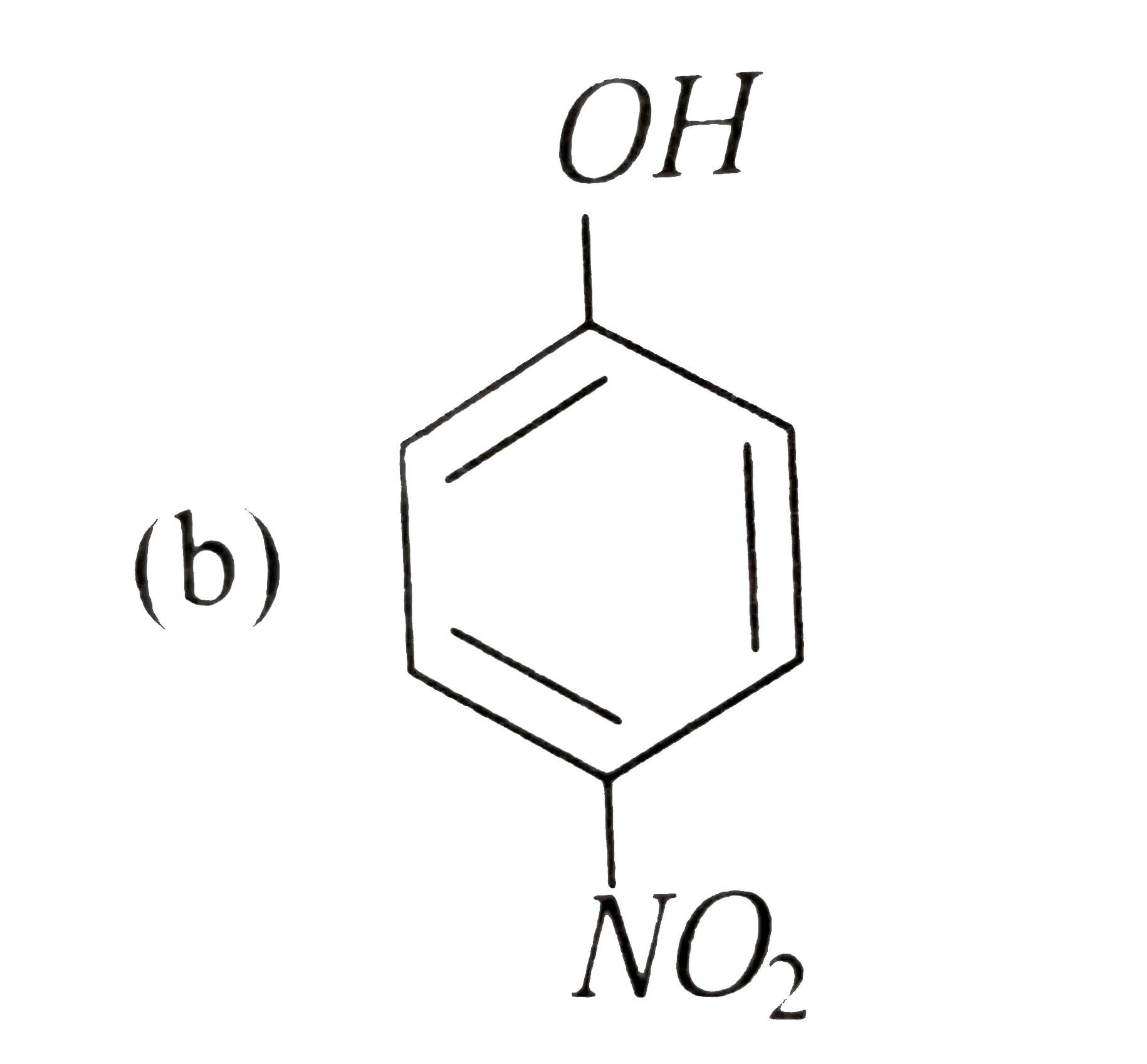 CC2H2DBoth (a) and (c)
CC2H2DBoth (a) and (c)Phenol is more acidic than :
AC2H5OHBCH3COOHC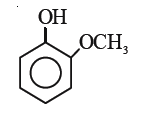 D
D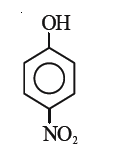
Phenol is more acidic than alcohol because :
Aphenol is more soluble in polar solventsBalcohol does not lose hydrogen atomCphenoxide ion is stabilised by resonanceDphenoxide ion does not exhibit resonance
Why is carboxylic acid a stronger acid than phenol ?
View SolutionPhenol is more acidic than ethanol.
View SolutionWhy are carboxylic acids called fatty acids?
View SolutionCarboxylic acids are more acidic than phenol and alcohol because of
View SolutionPhenol are more acidic than alcohols because
View Solution

