(a) Write the steps and conditions involved in the following conversions :
(i) Acetophenone to 2-phenyl-2-butanol
(ii) Propene to acetone.
(b) Describe simple chemical tests to distinguish between the following pairs of compounds :
(a) (i) Acetophenone to 2-Phenyl-2-butanol :

( b) Add sodium metal. Diethyl ether will not react. Propanol will react to form hydrogen gas.
CH3CH2CH2OH+Na→CH3CH2CH2Ona+1/2H2↑⏐Sodium propanoate
Similar Questions
Give a chemical test to distinguish between the following pairs of compounds:
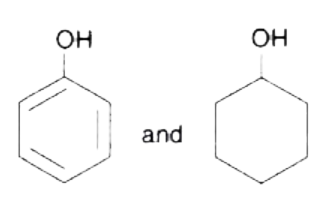
View SolutionWrite the steps and conditions involved in the following conversions:
acetophenone to 2-phenyl-2-butanol.
View SolutionWrite the steps and conditions involved in the following conversions:
Propyne to acetone.
View SolutionGive simple chemical tests to distinguish between the following pairs of compounds :
(a) Ethanal and Propanal
(b) benzoic acid and phenol
View SolutionGive simple chemical tests to distinguish between the following pairs of compounds :
(a) Ethanal and Propanal
(b) benzoic acid and phenol
View SolutionGive simple chemical tests to distinguish between the following pairs of compounds :
(a) Ethanal and Propanal
(b) benzoic acid and phenol
View Solution(a) Write the products of the following reactions :
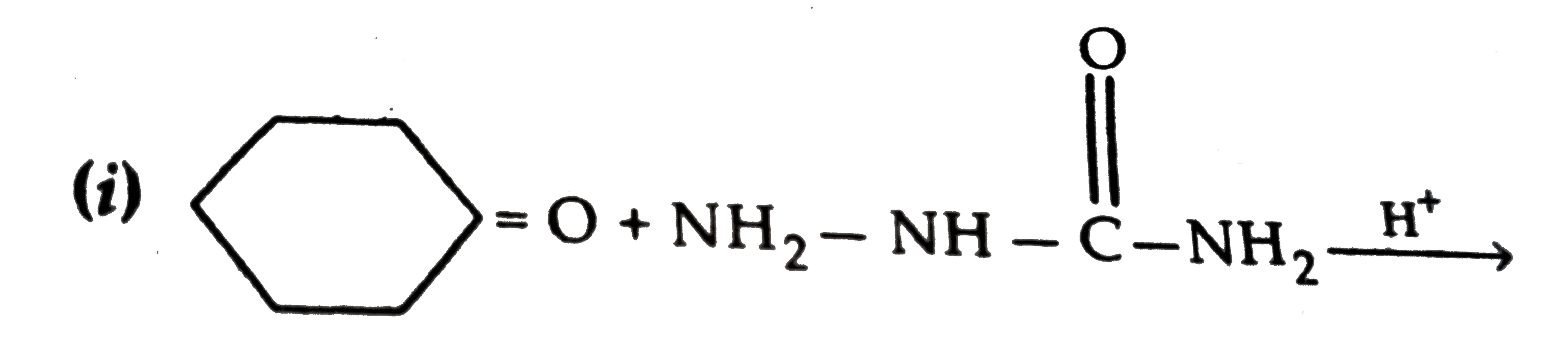
(ii) CH3MgBr+CO2Dry ether−−−−−→H3O+
(iii) CH3CH2COOH+Br2Red Phosphorus−−−−−−−−−→
(b) Write simple chemical tests to distinguish between the following pairs of compounds :
(i) Propanal and propanone (ii) Benzaldehyde and Benzoic acid
View SolutionGive chemical tests to distinguish between the following pairs of compounds :
Propanal and propanone.
View SolutionGive chemical tests to distinguish between the following pairs of compounds :
Propanal and propanone.
View SolutionGive simple chemical tests to distinguish between the following pairs of compounds:
Propanal and diethyl ether.
View Solution

