Write Lewis dot structure of CO molecule.
(i) C [He] 2s22p2, So valence electron = 4
O [He] 2s22p4, So valence electron = 6
∴ Total valence electron in CO = 10
(ii) The skeletal structure of CO is written as C - O .
(iii) Draw a single hnnci (one shared electron pair) between C and O and complete the octet on O, the remaining two electrons are the lone pair on C.
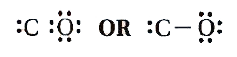
OR :C=←−−O: Lewis structure × Electron of C
(iv) This does not complete the octet on carbon and hence we have to resort to multiple bonding (in this case a triple bond) between C and O atoms. This satisfies the octet rule condition for both atoms.

C and O has triple bond. Two covalent bond one coordinate bond.
Note: Triple bond is three. One coordinate bond and two covalent bond between C and O. In co- ordinate bond both electron is donated by oxygen.
Topper's Solved these Questions
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:KUMAR PRAKASHANChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:TRY YOUR SELF-3
Explore 1 Video
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:KUMAR PRAKASHANChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:TRY YOUR SELF-4
Explore 1 Video
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Book:KUMAR PRAKASHANChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Exercise:Section -D SOLUTIONS OF NCERT EXEMPLAR PROBLEMS (Long Answer Type Questions )
Explore 7 Videos
Similar Questions
In Cl2 molecule covalent radius is 99 pm and van-dar-Walls radius is 180 pm what is the distance between two nucleus and distance between two molecules ?
View SolutionWrite only arrangement of electron and its shape of the following.
HgCl2 (ii) BF3 (iii) NH+4(iv)PCl5
(v) SF6 (vi) SO2 (vii)NH3 (viii)H2O
(ix)SF4 (x)CIF3 (xi)BrF5 (xii)XeF4
View SolutionDiscuss the factors affecting electron gain enthalpy and the trend in its variation in the periodic table.
View SolutionWrite the Lewis dot structure of CO molecule .
View SolutionWrite the Lewis dot structure of CO molecule .
View SolutionCO के अणु की लूइस बिंदु संरचना लिखें |
View SolutionWrite the lewis dot structure of CO2−3 molecule.
View SolutionWrite Lewis dot structure of HCOOH .
View SolutionWrite the Lewis dot structure of CO molecule .
View SolutionWrite the Lewis structure for CO molecule.
View SolutionWrite the Lewis dot structure of CO molecule.
View SolutionWrite the Lewis dot structures for the following compounds: NaCN, N2O, CO, COCl2
View SolutionWrite the Lewis dot structure of CO molecule .
View SolutionWrite the Lewis dot structure of the following molecules or species: C2H2
View SolutionWrite the Lewis dot structure of the following molecules or species: PCI3
View Solution

