Identify the wrong statement from the statements given below.
A
Geometrical structwe of BrF5 is square pyramidal.
B
Bond order and Bond energy of a molecule are directly related
C
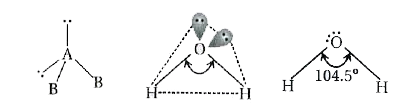
H-O-H bond angle in water molecule is 104∘.30' because O atom is sp3 hybridized.
D
Strength of σ bond is related to the magnitude of overlap of atomic orbitals.
Text Solution
The correct Answer is:C
H-O-H bond angle in water molecule is 104∘.30. because O atom is sp3 hybridized.
This statement is wrong. As .O. has sp3 hybridization but due to repulsion between lone pair on oxygen bond angle decrease from 109∘28. and become 104∘30. .
|
Similar Questions
Identify the wrong statement from the below.
View SolutionIdentify the wrong statement from the below.
View SolutionIdentify the wrong statement from the below.
View SolutionIdentify the wrong statement from the below
View SolutionIdentify the wrong statements from the below.
View SolutionIdentify the wrong statement from the below
View SolutionIdentify the wrong statements from the below.
View SolutionIdentify the wrong statement from the below
View Solution

