Why does type or overlap given in the following figure not result in bond formation ?
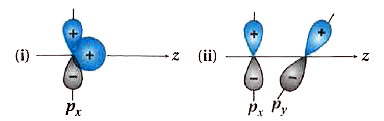
Text Solution
ln the figure (I), area or+ + overlap is equal to + - overlap, so net overlap is zero, while in figure (II), there is no overlap due to different symmetry.
|
Similar Questions
Knowledge Check
Which type of overlapping reusults in the formation of a π bond?
AAxial overlapping of s-s orbitalsBLateral overlapping of p-p orbitalsCAxial overlapping of p-p orbitalsDAxial overlapping of s-p orbitalsWhich of the following set of overlap can not provide π -bond formation
A3 d and 2pB2p and 3pC2p and 2pD3p and 1sWhich type of reaction is shown by the following figure
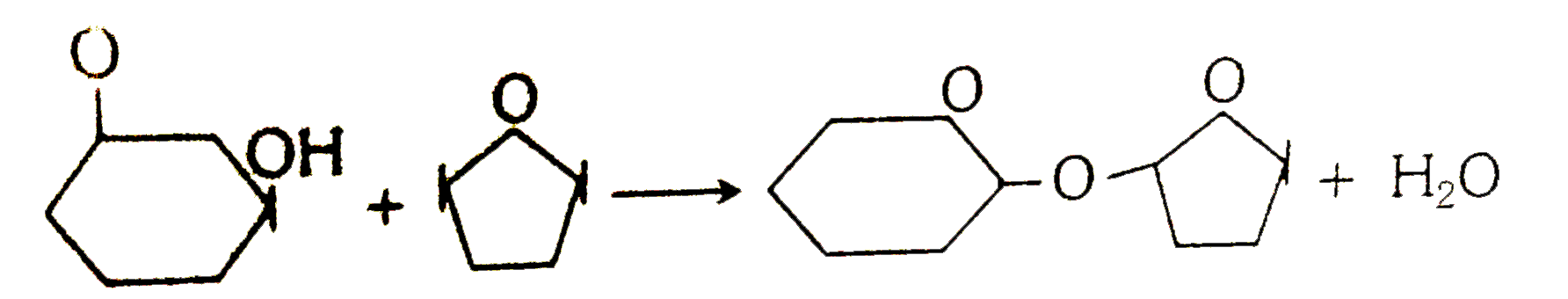
Or
Formation of both peptide and glycosidic bonds involvesAHydrationBDenaturationCDehydrationDDydrolysis
Why does type of overlap given in the following figure not result in bond formation?
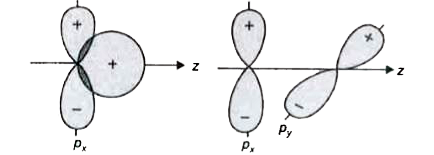
View SolutionWhy does type of overlap given in the following figure not result in bond formation?
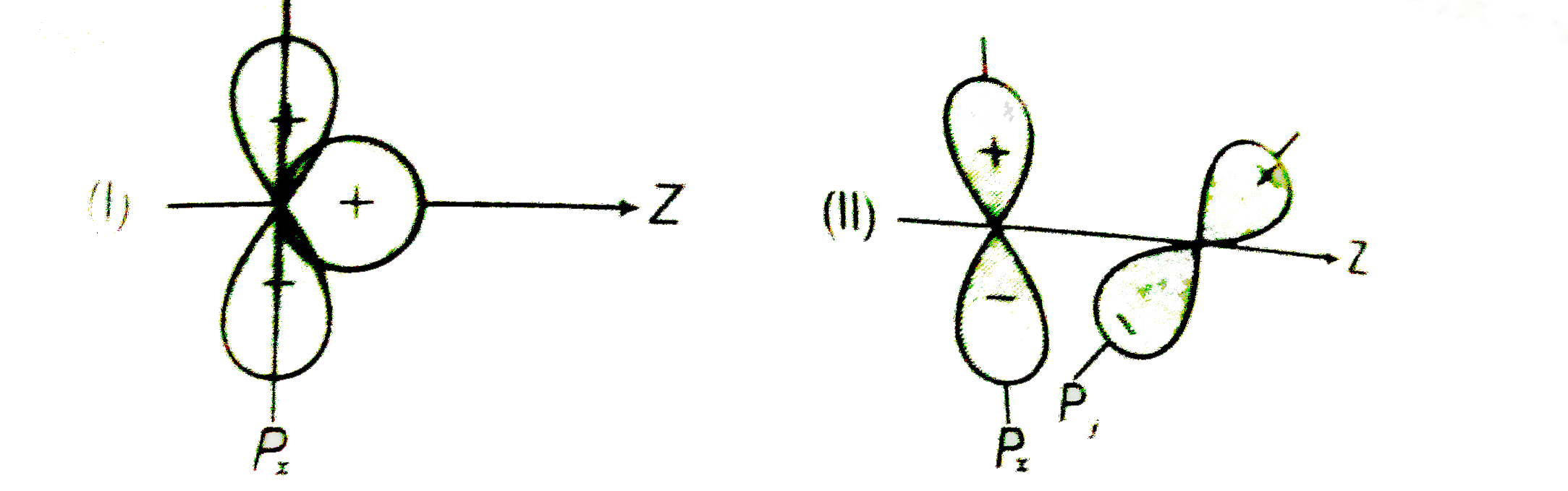
View SolutionWhy does type of overlap given in the following figure not result in bnd formation ?
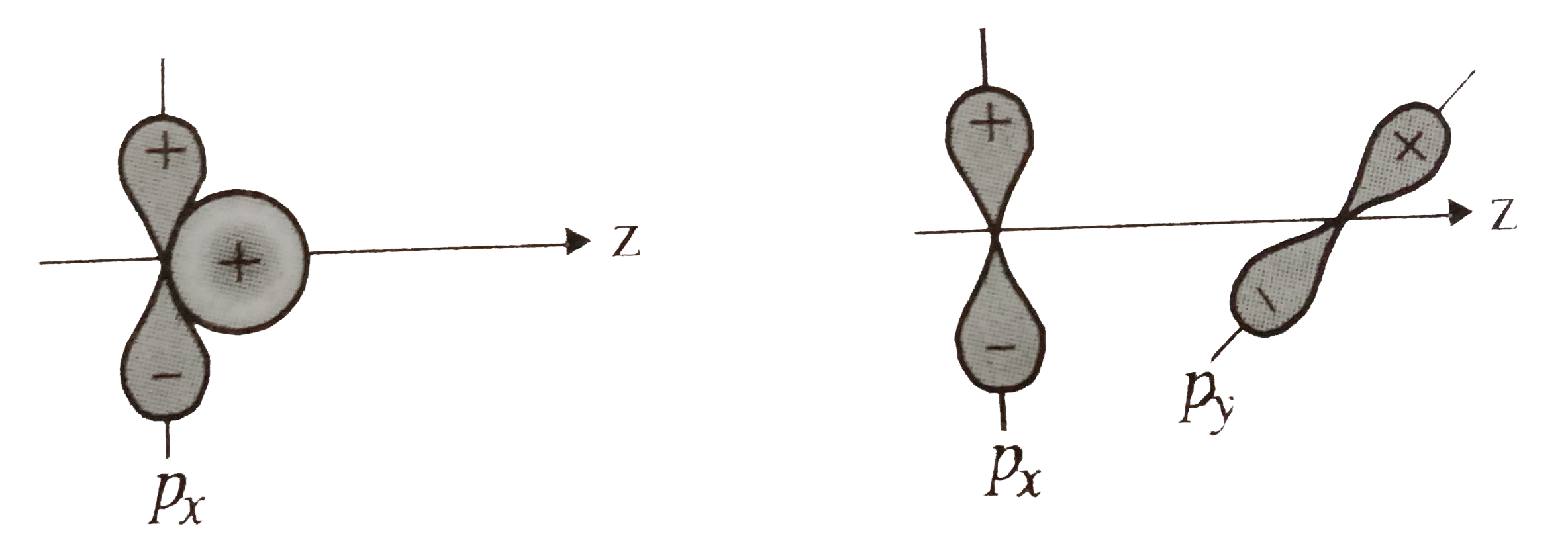
View SolutionWhich of the following set of overlap can not provide π -bond formation
View SolutionWhich one of the following reactions will not result in the formation of carbon - carbon bond?
View Solution

