Water molecule has bent structure whereas carbon dioxide molecule is linear
In H2O, oxygen atom is sp3 hybridised with two lone pairs. The four sp3 hybridised orbitals possess a tetrahedral geometry with two comers occupied by hydrogen atoms and other two by the lone pairs.
The bond angle is reduced to 104,5∘ from 109.5∘ because of greater repulsive forces between lp - Ip and the molecule so gain V-shape or bent structure (angular structure).
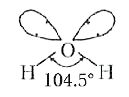
In CO2 molecule, carbon atom is sp-hybridised. The two sp hybrid orbitals are oriented in opposite direction forming an angle of 180∘.
So H2Omolecule has bent structure and CO2 molecule is linear.
Similar Questions
Give reasons for the following :
(i) Covalent bonds are directional while ionic bonds are non-directional.
(ii) Water molecule has bent structure whereas carbon dioxide molecule is linear.
(iii) Etyne molecule is linear.
View SolutionGive reason for the following.
a) Covalent bonds are directional bonds while ionic bonds are non-directional.
b) Wate molecules has bent whereas carbon dioxide molecule is linear.
c) Ethyne molecule is linear.
View Solution
Knowledge Check
The molecule havi linear structure is
AMethaneBEthyleneCAcetyleneDWaterThe molecule that has linear structure is:
ACO2BNO2CSO2DSiO2How many double bonds are present in carbon dioxide molecule?
AOneBTwoCThreeDFour
Give reason for the following.
a) Covalent bonds are directional bonds while ionic bonds are non-directional.
b) Wate molecules has bent whereas carbon dioxide molecule is linear.
c) Ethyne molecule is linear.
View SolutionThe molecule that has linear structure is:
View SolutionThe molecule has........... structure
View SolutionThe molecule that has a linear structure is :
View SolutionChlorophyll molecules has the structure like
View Solution

