Elements X, Y and Z have 4, 5 and 7 valence electrons respectively,
(a) Write the molecular formula of the compounds formed by these elements individually with hydrogen.
(b) Which of these compounds will have the highest dipole moment ?
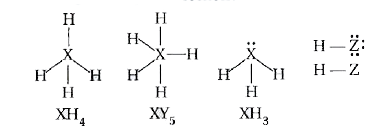
Z has seven electrons in its valence shell. It is the most electronegative element. Therefore, HZ will have the highest dipole moment.
Similar Questions
Elements X,Y and Z have 4,5 and 7 valence electrons respectively, (i) Write the molecular formula of the compounds formed by these elements individually with hydrogen (ii) which of these compounds will have the highest dipole moment ?
View SolutionElements X,Y and Z have 4,5 and 7 valence electrons respectively, (i) Write the molecular formula of the compounds formed by these elements individually with hydrogen (ii) which of these compounds will have the highest dipolw moment ?
View Solution
Knowledge Check
Which of the following compounds will have the highest dipole moment ?
A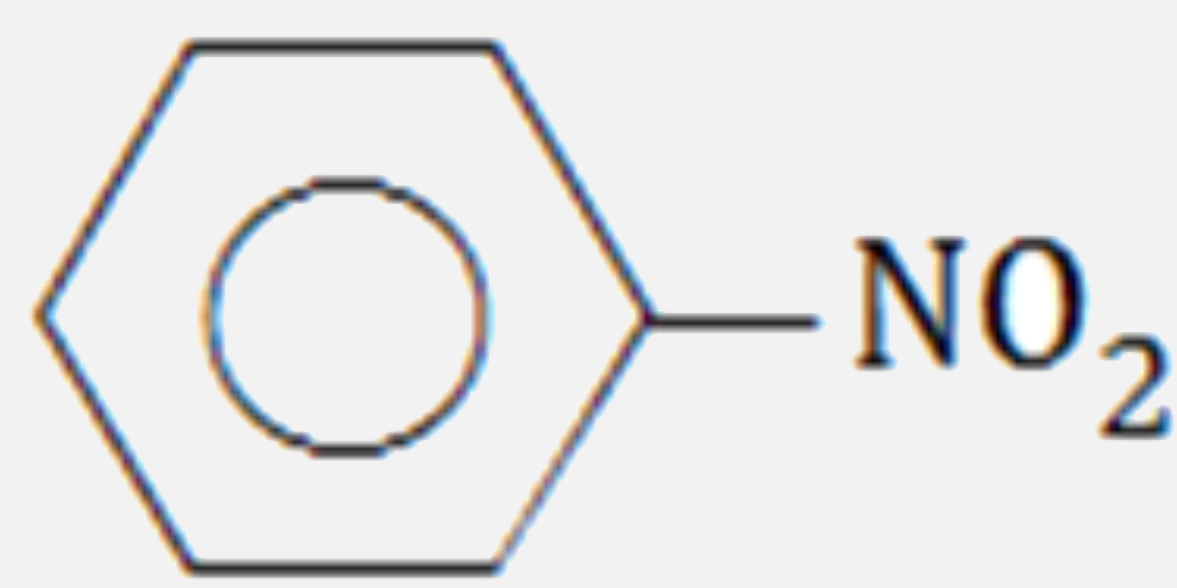 B
B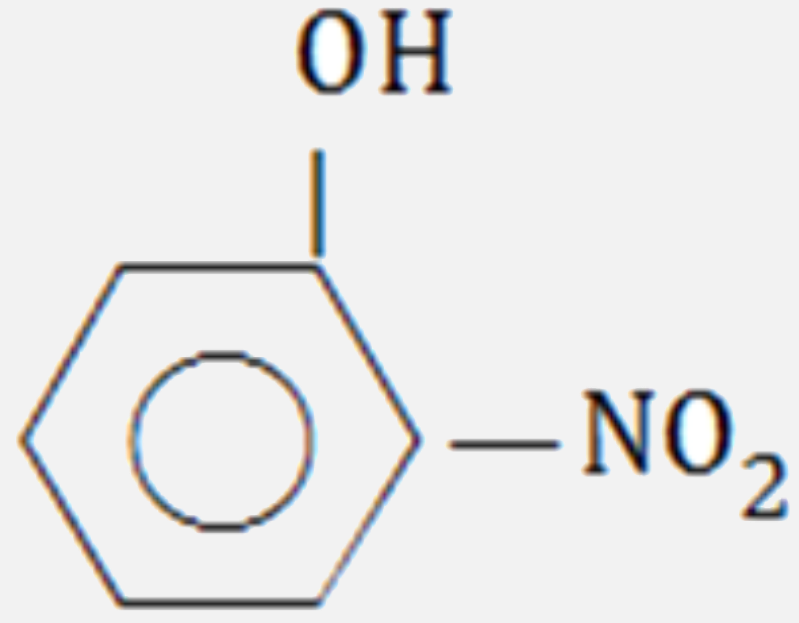 C
C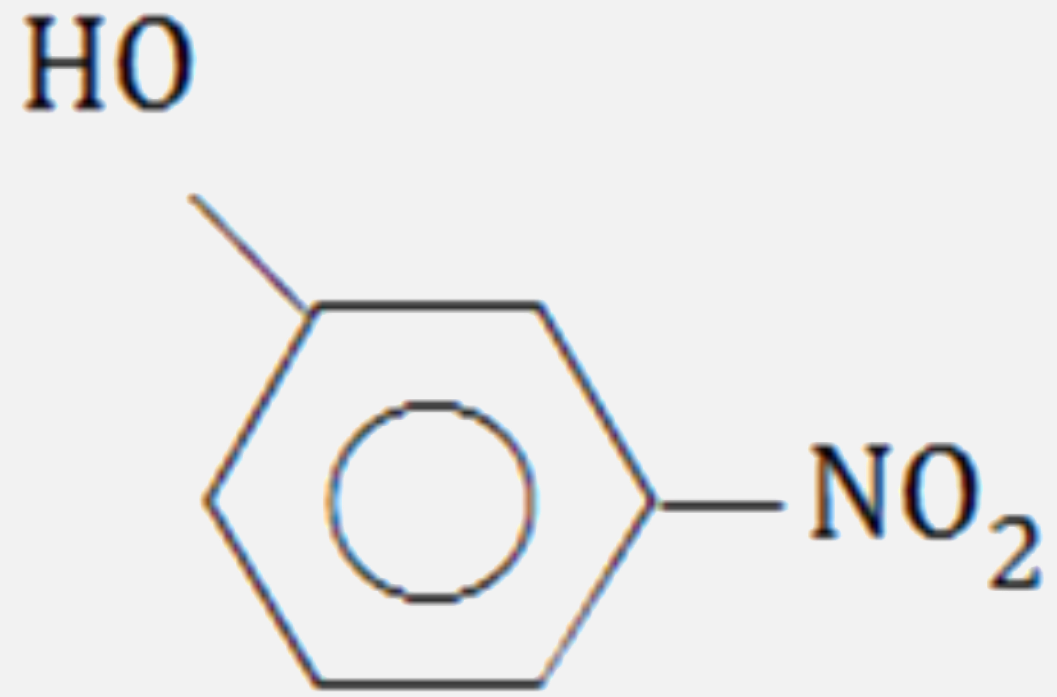 D
D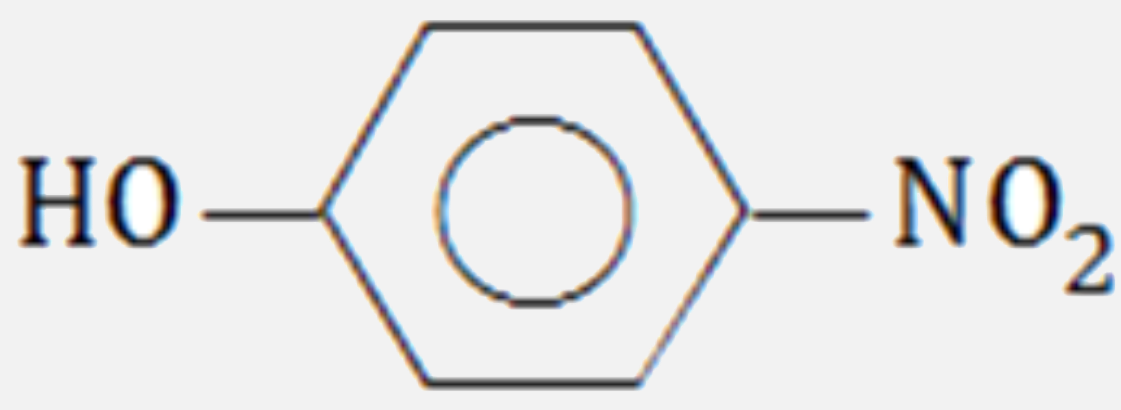
Elements X,Y and Z have 4,5 and 7 valence electrons respectively, (i) Write the molecular formula of the compounds formed by these elements individually with hydrogen (ii) which of these compounds will have the highest dipolw moment ?
View SolutionElements X,Y and Z have 4,5 and 7 valence electrons respectively, (i) Write the molecular formula of the compounds formed by these elements individually with hydrogen (ii) which of these compounds will have the highest dipolw moment ?
View SolutionWhich of the following compounds will have the highest dipole moment ?
View SolutionThe two elements X and Y have 5 and 7 valence electron respectively, what will be the formula of the compound formed by them?
View SolutionElements A, B and C have atomic numbers 9, 20 and 10 respectively.
Write down the formula of the compound formed by two of the above elements.
View SolutionThree elements X, Y and Z have atomic numbers 7, 8 and 9 respectively.
Write the formula of compound formed between
X and Z
View SolutionThree elements X, Y and Z have atomic numbers 7, 8 and 9 respectively.
Write the formula of compound formed between
X and Y
View Solution

