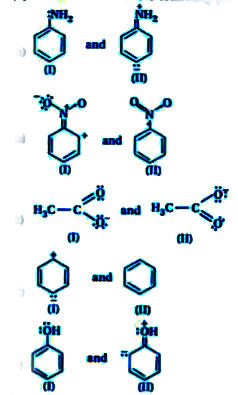
(i) Identify less stable resonance structure and (ii) Give its reason in the following pairs.
Text Solution
(a) Structure (II) is less stables because there is the separation of charge in it.
(b) Structure (I) is less stable because there is the sepration of charge in it
(c ) Structure (I) and (II) are equal stable because both have same structure.
(d) Structure (I) is less stable because there is the separation of charge in it.
(e ) Structure (II) is less stable because there is the separation of charge in it.
|
Similar Questions
Knowledge Check
The most stable resonating structure is
AH2N−⊕CH−CH=CH−OCH3BH2⊕N=CH−CH=CH−OCH3C DNH_(2)-overset(o+)(C)H-overset(ɵ)(C)H-CH=overset(o+)(O)CH_(3)
DNH_(2)-overset(o+)(C)H-overset(ɵ)(C)H-CH=overset(o+)(O)CH_(3)Which is the most stable resonating structure ?
A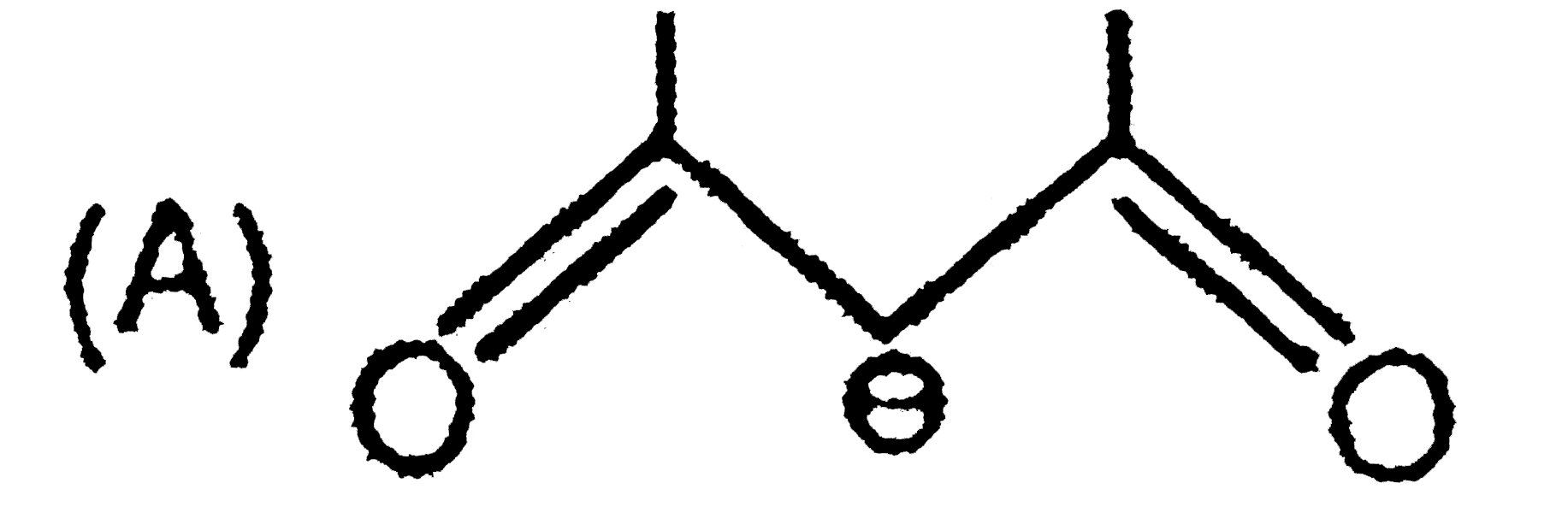 B
B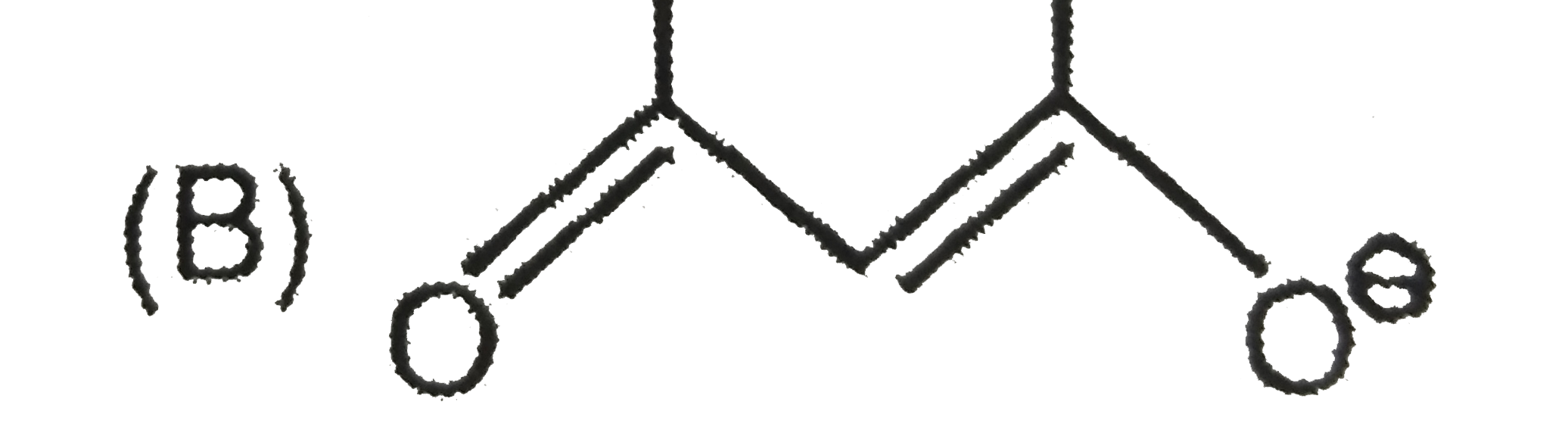 C
C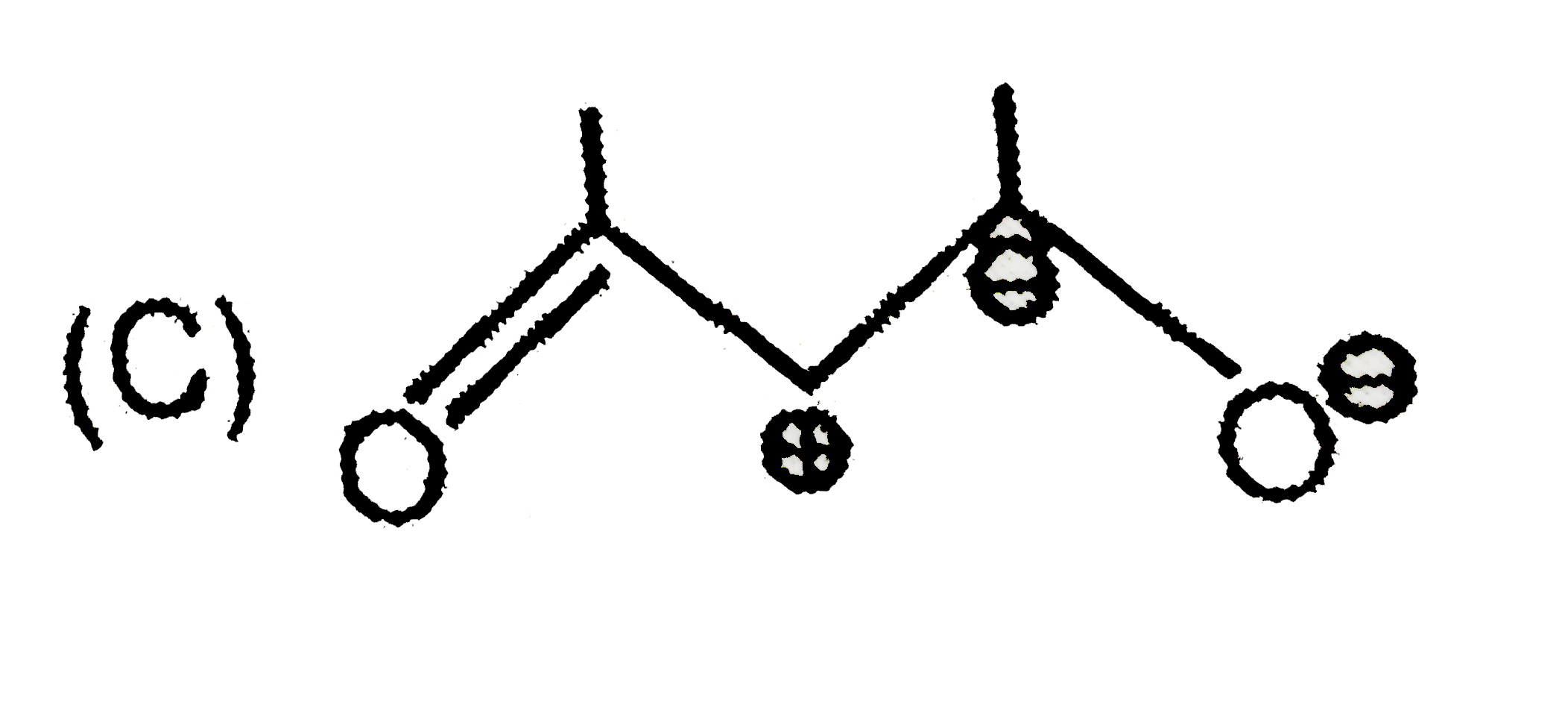 D
D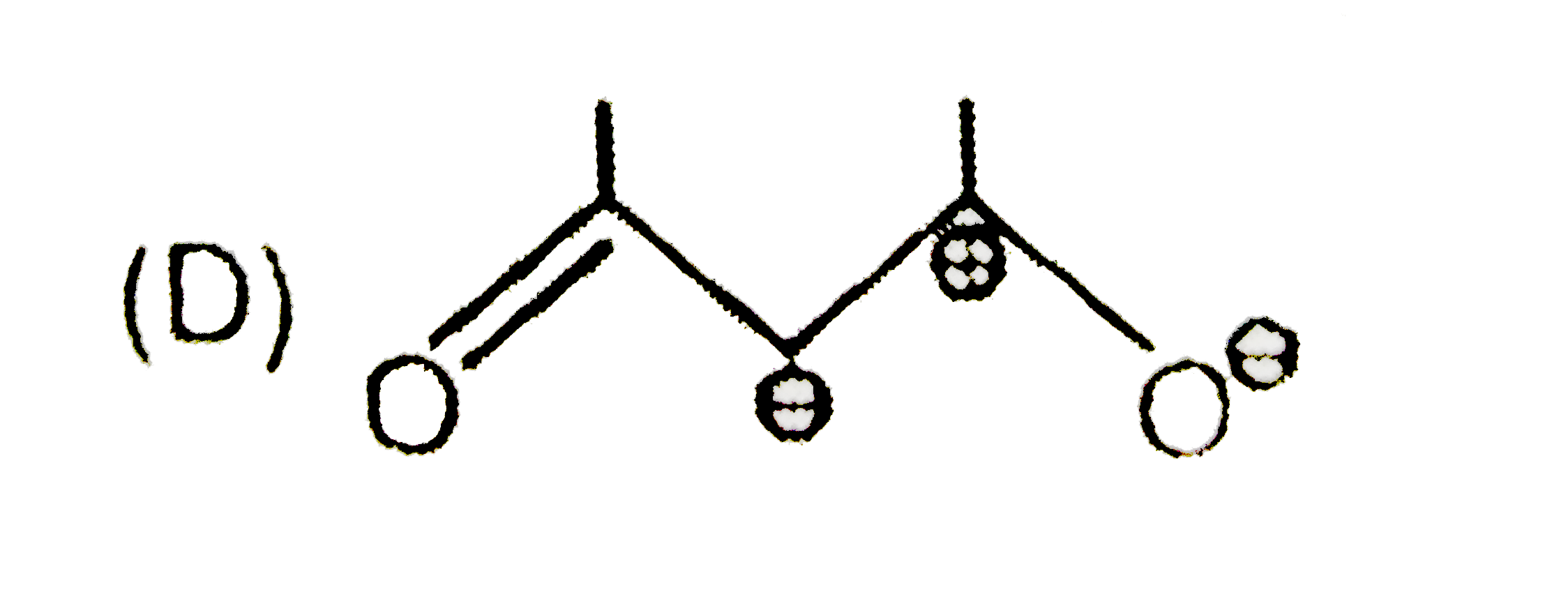
Which will be least stable resonating structure?
ACH_(2)=CH-overset(oplus)(C)H-overset(ө)(C)H-O-CH_(3)BөH2−⊕H−CH=CH−OCH3C⊕H2−CH=CH−CH=⊕O−CH3DCH2=CH−өCH−CH=⊕O−CH3
Which will be the least stable resonating structure :
View SolutionWhich will be the least stable resonating structure ?
View SolutionAmong the following, the least stable resonance structure is :
View SolutionAmong the following, the least stable resonance structure is :
View SolutionAmong the following, the least stable resonance structure is :
View Solution

