Write equations for the steps in SN1 mechanism of conversion of tertiary butyl bromide into tertiary butyl alcohol. Solution in Kannada
a) The mechanisminvolves the following two steps.
Step 1:
Formation of carbocation: t-butyl bromide undergoes ionization to form a t-butyl carbocation.
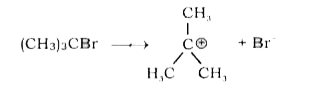
This step is the slowest stephence it is the rate determining step.
Step-2:
Attack of nucleophile on the carbocation : The nucleophile OH− can attack the carbocation either from front side or back side to form t - butyl alcohol. The step is fast.
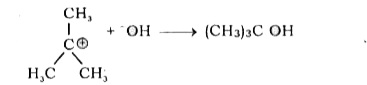
∴ Rate of the reaction is directly α [t - butyl bromide]1[OH−]0
∴ Order = 1
Similar Questions
Write the equations for the steps in S_(N)1 mechanism of the conversion of tert-Butyl bromide into tert-Butyl alcohol. (a) Explain Fitting reaction. (b) Name the reagent used in the dehydrohalogenation of haloalkanes.
View SolutionWrite the equations for the steps in SN1 mechanism of the conversion of tert-Butyl bromide into tert-Butyl alcohol.
(a) Explain Fitting reaction.
(b) Name the reagent used in the dehydrohalogenation of haloalkanes.
View Solutionतृतीयक ब्यूटिल एल्कोहल किसके साथ अभिक्रिया करके तृतीयक ब्यूटिल क्लोराइड देता है
View SolutionTertiary butyl alcohol gives tertiary butyl chloride on treatement with
View SolutionWrite equations for the steps in SN1 mechanism of conversion of tertiary butyl bromide into tertiary butyl alcohol.
View Solutiona) i) write the equations for the steps in SN1 mechanism of the conversion of tertiary butyl bromide in to tertiary butyl alcohol.
ii) Haloarenes are less reactive towards nucleophilic substitution reactions than Haloalkanes. Give a reason.
View SolutionWrite steps involved in S_(N)1 mechanism to the conversion of tertiary butyl bromide into tertiary butyl alcohol and mention its order.
View SolutionConversion of Acetic acid to acetone and tertiary butyl alcohol
View Solution
