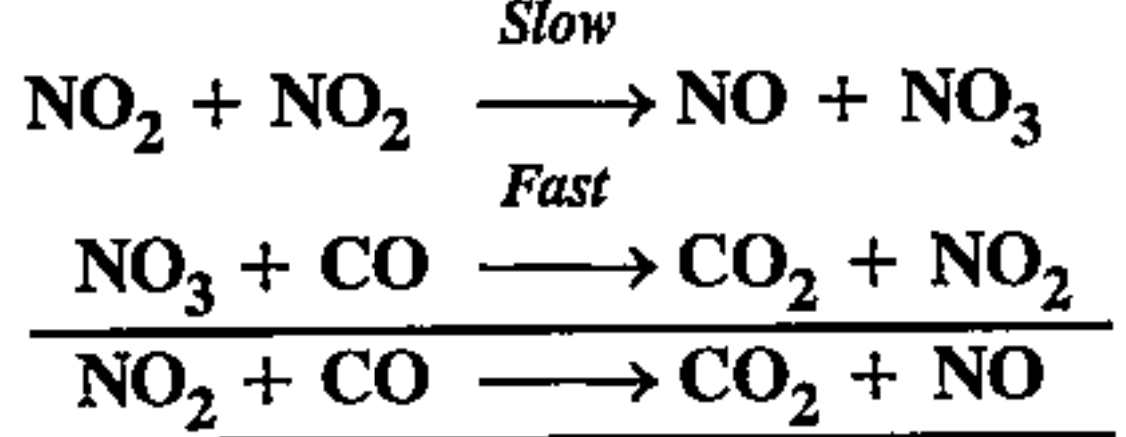
Reaction between NO2 and CO to give CO2 and NO takes place by the following mechanism:
Write the rate expression and order of the reaction. What is the unit of rate constant ?
 Solution in Punjabi
Solution in Punjabi
Step by step video & image solution for Reaction between NO_2 and CO to give CO_2 and NO takes place by the following mechanism: Write the rate expression and order of the reaction. What is the unit of rate constant ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Derive an expression for rate constant of 1st order reaction. Write the unit of K.
View SolutionSignificance OF Order OF Reaction || Expression Determination OF Order OF Reaction and unit OF Rate Constant
View SolutionFactor affecting Rate OF Reaction||Rate Law Expression||Order OF Reaction|| Unit OF Rate Constant|| Determination OF Order from unit OF Rate Constant
View SolutionFrom the rate expression for the following reaction determine the order of reaction and dimensions of the rate constant. 3NO(g)→N2O(g)+NO2(g),Rate=K[NO]2.
View SolutionFor the reaction NO_2(g) +CO (g) to CO_2 (g) +NO (g) the experimentally determined rate expression below 400 K is: rate =k [NO_2]^2 . What mechanism can be proposed for the above reaction ?
View SolutionFor the reaction
NO2(g)+CO(g)→CO2(g)+NO(g)
the experimentally determined rate expression below 400 K is: rate =k[NO2]2. What mechanism can be proposed for the above reaction ?
View SolutionFor the reaction
NO2(g)+CO(g)→CO2(g)+NO(g)
the experimentally determined rate expression below 400 K is: rate =k[NO2]2. What mechanism can be proposed for the above reaction ?
View SolutionReaction between NO2 and COto give CO2takes place by the following mechanism :
NO2+NOSlow−−→NO+NO3
NO3+COFast−−→NO2+CO2
Write the rate expression and order of the reaction.
View Solution

