The addition of HCl does not suppresses the ionisation of :Solution in Odia
Acetic acid
Benzoic acid
H2S
H2SO4
The correct Answer is:D
Step by step video, text & image solution for The addition of HCl does not suppresses the ionisation of : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
The addition of HCl in presence of peroxide does not follow anti-Markovnikov's rule because
View SolutionStatement: The pH of an aqueous solution of acetic acid remains unchanged on the addition of sodium acetate.
Explanation: The ionisation of acetic acid is suppressed by the addition of sodium acetate.
View SolutionThe dissociation of H2S is suppressed in the presence of HCl. Name the phenomenon.
View SolutionThe dissociation of HCN is suppressed by the addition of HCI. Explain.
View SolutionAddition of Hl will significantly suppress solubility or dissociation or hydrolysis of which of the following ?
View SolutionThe preparation steps involved in the free radical addition of HX across a double bond are:
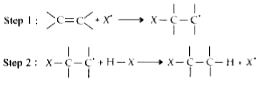
HCl does not follow free radical addition
View SolutionThe addition of HCl will not supress the ionisation of
View Solution
