Complete the reaction as follows.
Complete oxidation of methane :
CH4(g)Methane+2O2(g)→CO2(g)+2H2O(l)ΔcHɵ=−890kJmol−1
Topper's Solved these Questions
- HYDROCARBONSBOOK - KUMAR PRAKASHANCHAPTER - HYDROCARBONSEXERCISE - SECTION - A
55 Videos
- HYDROCARBONSBOOK - KUMAR PRAKASHANCHAPTER - HYDROCARBONSEXERCISE - Section - B objective questions (short questions)
147 Videos
- EQUILIBRIUMBOOK - KUMAR PRAKASHANCHAPTER - EQUILIBRIUMEXERCISE - Question Paper from Module
7 Videos
- HYDROGENBOOK - KUMAR PRAKASHANCHAPTER - HYDROGENEXERCISE - Section-D NCERT Exemplar (Long Answer)
8 Videos
Similar Questions
Write structures of different chain isomers of alkanes corresponding to the molecular formula C6H14. Also write their IUPAC names.
View SolutionGive reaction for the formation of butane from ethane.
View SolutionAt 60∘H2O(l)−−→←−−H2O(g) in this equilibrium the value of Kp ? At 60∘C upper pressure of water is 0.185 bar.
View SolutionAtomic hydrogen combines with almost all elements but molecular hydrogen does not. Explain.
View SolutionComplete the following reactions :
(a)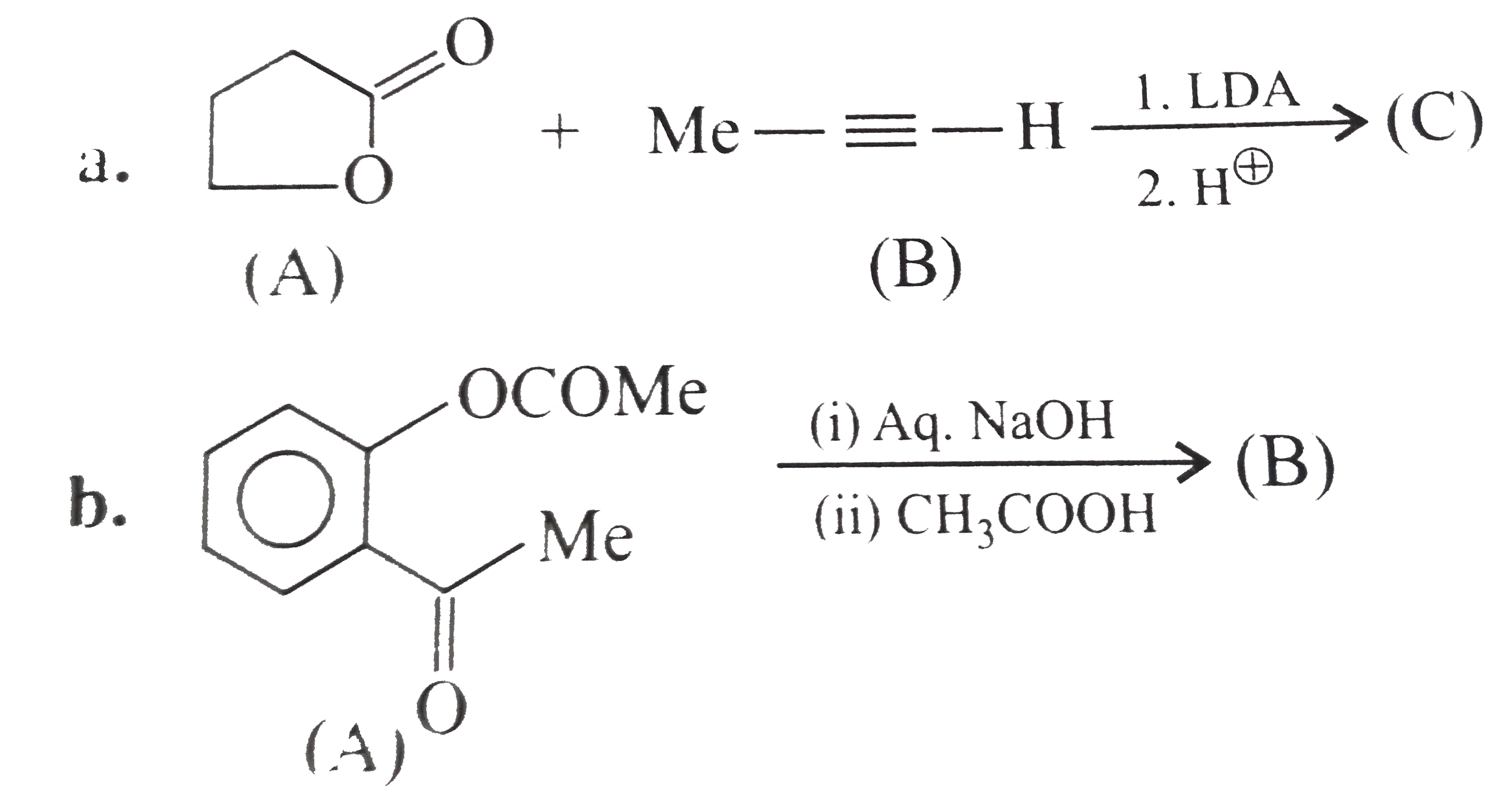
View SolutionComplete the following reactions:
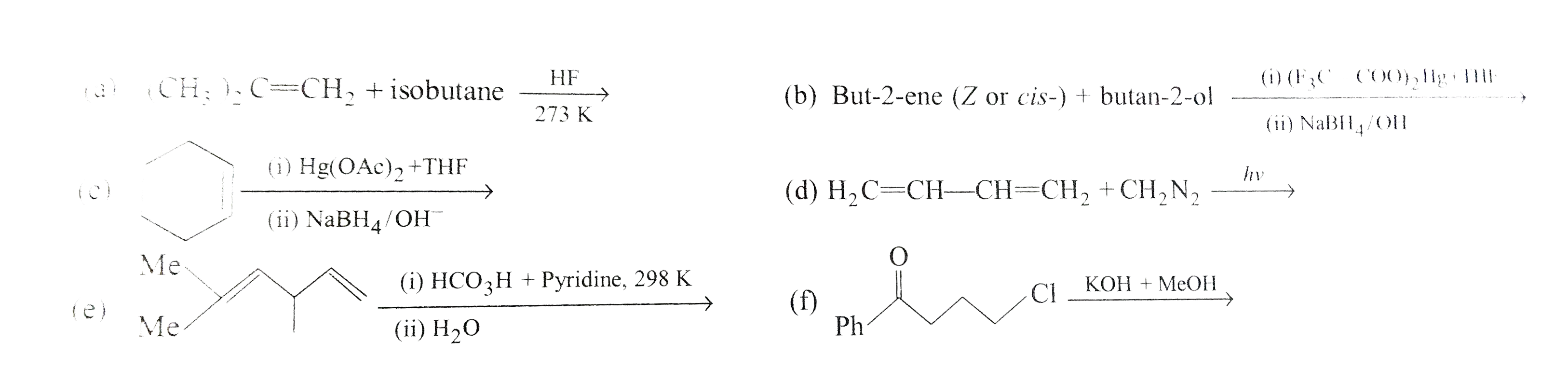
View SolutionComplete the following reactions

View SolutionAmong the following oxidation reactions of methane , which two are controlled oxidation reactions
View SolutionComplete the oxidation reaction. CH4+2O2→_____+______.
View SolutionThe volume of oxygen required for complete oxidation of 2.0 litre methane at NTP is :
View SolutionComplete the reaction as follows.
Complete oxidation of butane :
View SolutionComplete the reaction as follows.
Complete oxidation of alkane :
View SolutionComplete the reaction as follows.
Incomplete combustion of methane :
View SolutionComplete the reaction as follows. Oxidation 2-methylpropane reaction :
View SolutionHow many oxidation reactions are involved in the complete oxidation of one glucose molecule?
View Solution

