In cis and trans, whose dipole moment is more, explain with example ?
Text Solution
CH3CH=CHCH3 has 2 geometrical isomers :
(i) Cis-but-2-ene (ii) Trans-but-2-ene.
Always cis-isomer dipole moment (μ)> Trans-isomers.
Isomers of But-2-ene are shown with its isomers and dipole moment.
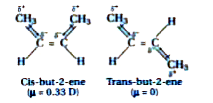
Trans-but-2-ene has 2−CH3 group, they are in opposite side therefore dipole moments of C−CH3 bonds circle, thus making the trans form non-polar.
Cis isomers are polar because in Cis-but-2-ene has 2−CH3 group and they are on same side of double bond. Electronegativity of CCH3 are added, in which the dipole moment is more. therefore, cis-isomer is polar and trans isomer is non-polar.
|
Similar Questions
Knowledge Check
Cis - and trans - faces are present in
AEndoplasmic reticulumBGolgi bodyCRibosomesDMitochondriaCis and trans isomers generally
Acontain a triple bondBcontain double bonded carbon atomsCare enantiomersDrotate the plane of plane-polarized lightAssertion: cis-form of alkene is found to be more polar than the trans-form.
Reason: Since the groups are in opposite directions in the trans-form, the dipole moments of bonds cancel each other making trans-form almost non-polar.AIf both assertion and reson are true and reason is the correct explanation of assertionBIf both assertion and reason are true but reason is not the correct explanation of assertion.CIf assertion is true but reason is false.DIf both assertion and reason are false.
What is the importance of Trans continental roads? Explain with examples.
View SolutionWrite true or false. Cis-trans isomers have different dipole moments
View SolutionAssertion: cis-form of alkene is found to be more polar than the trans-form.
Reason: Since the groups are in opposite directions in the trans-form, the dipole moments of bonds cancel each other making trans-form almost non-polar.
View SolutionDipole moment is possessed by (one or more)
View SolutionCis-and trans2-butane are
View Solution

