Give the physical properties of alkene ?
Text Solution
Physical properties : Alkenes as a class resemble alkanes in physical properties, except in types of isomerism and difference in polar nature.
Physical conditions : The first three members are gases, the next fourteen are liquids and the higher ones are solids, Ethene is a colourless gas with afaint sweet. All other alkenes are colourless and odouless, insoluble in water but fairly soluble in nonpolar solvents like benzene, petroleum ether.
Boiling point : As there is increase in size and molecular weight of alkene, their is increase in boiling point. On adding −CH2 group, boiling point increases 20-30 K.
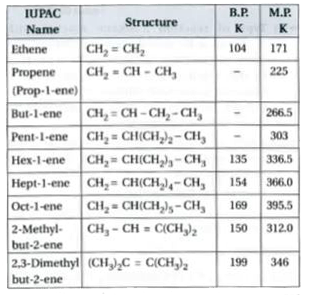
Boiling point and melting point alkene are higher than alkanes.
Chain isomer of alene boiling point are higher than the branched isomer of alkene.
Boiling point of trans isomer is higher than cis and melting of trans isomer is less than cis isomer.
|
Topper's Solved these Questions
- HYDROCARBONSBook:KUMAR PRAKASHANChapter:HYDROCARBONSExercise:SECTION - A
Explore 55 Videos
- HYDROCARBONSBook:KUMAR PRAKASHANChapter:HYDROCARBONSExercise:Section - B objective questions (short questions)
Explore 147 Videos
- EQUILIBRIUMBook:KUMAR PRAKASHANChapter:EQUILIBRIUMExercise:Question Paper from Module
Explore 7 Videos
- HYDROGENBook:KUMAR PRAKASHANChapter:HYDROGENExercise:Section-D NCERT Exemplar (Long Answer)
Explore 8 Videos
Similar Questions
Give two physical properties of water.
View SolutionGive the physical properties and chemical composition of urine.
View SolutionPhysical Properties Of Metal
View SolutionGeneral physical properties of alchols.
View SolutionGive reasons
Physical properties of isotopes are different
View SolutionGive reasons for the following
The physical properties of isotopes are different.
View SolutionGive reasons for the following:
Isomers belonging to the same homologous series may differ in physical properties but not in chemical properties.
View SolutionDiscuss the important physical and chemical properties of chlorobenzene. Give its important uses.
View SolutionIdentify the statement which does not describe the properties of alkenes
View SolutionAlkenes || Chemical Properties OF Alkenes III
View Solution

