What is the rate of reaction with Cl2,Br2 and I2 with CH2≡CH2.
Reactivity order Cl2>Br2>I2. Rate of reaction of alkyne with iodine (I2) is so slow hence special condition is required to arrange equilibirum.
Similar Questions
Which compound in each of the following pairs will react faster in SN2 reaction with OH−?
(a) CH3Br or CH3I
(b) CH2=CHBr or CH2=CHCH2Br.
View SolutionWhich compound in each of the following paris will react faster in SN2 reaction with HOo− ?
a. CH_(3)BrorCH_(3)I<br>b.(CH_(3))_(3)CClorCH_(3)Cl<br>c.CH_(2) = CHBrorCH_(2) = CH - CH_(2)Br
View Solution
Knowledge Check
The reaction RCH2CH2COOHRedP+Br2−−−−−−→R−CH2−CH(Br)−COOH
AReimer- Tiemann reactionBHell-Volhard - Zelinsky reactionCCannizzaro reactionDSandmeyer reaction .Which compound in each of the following paris will react faster in SN2 reaction with HOo− ?
a. CH_(3)BrorCH_(3)I<br>b.(CH_(3))_(3)CClorCH_(3)Cl<br>c.CH_(2) = CHBrorCH_(2) = CH - CH_(2)BrA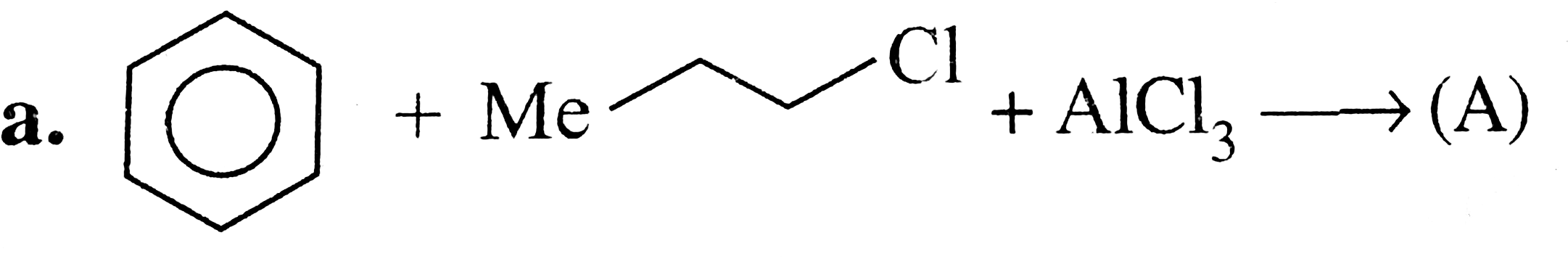 B
B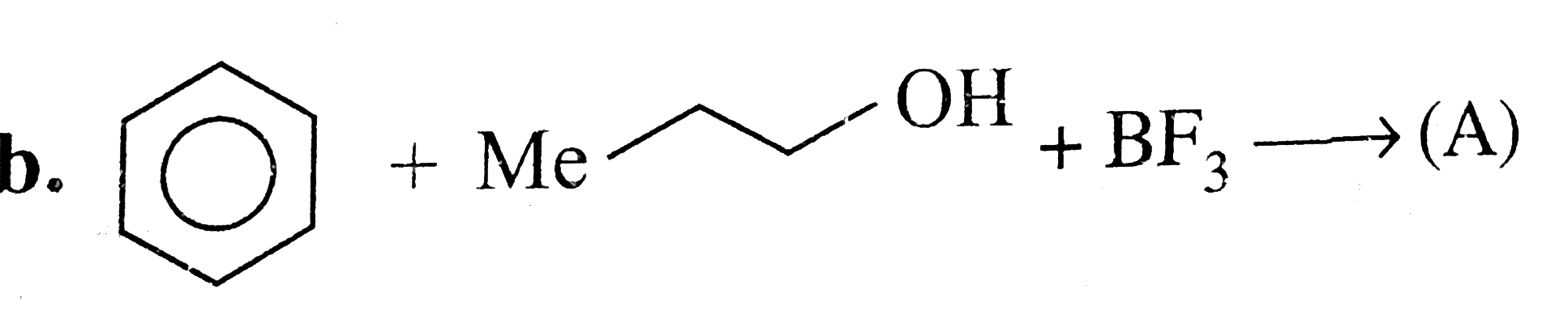 C
C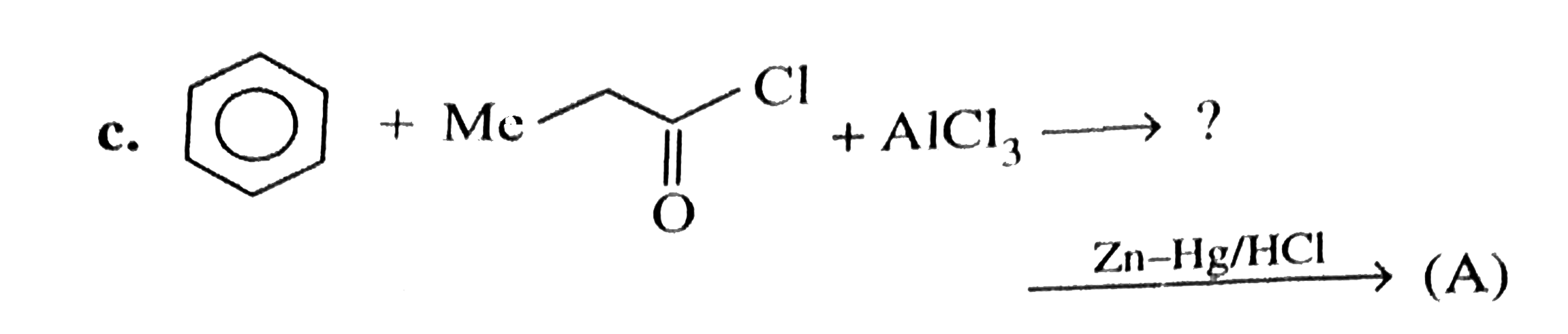 D
D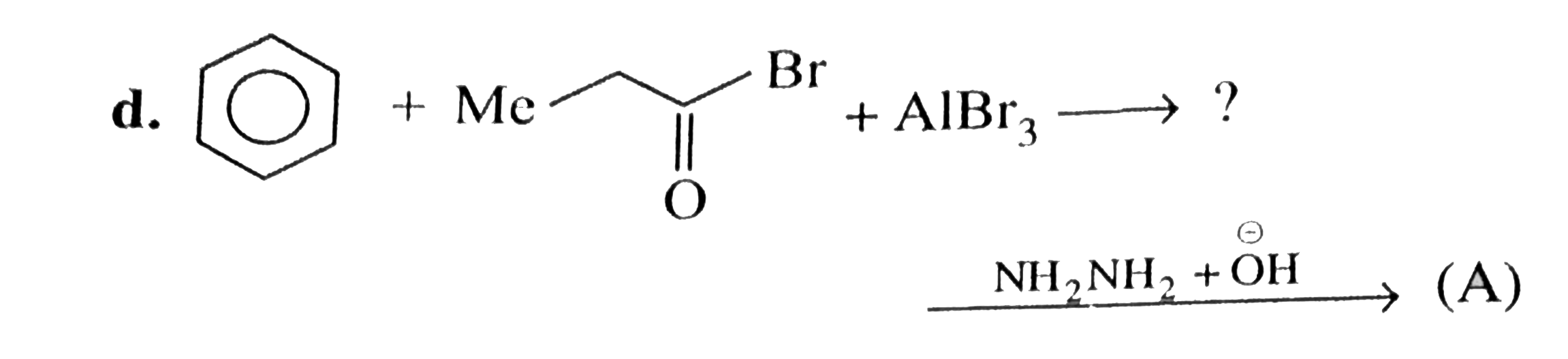
Decreasing order of reactivity in Williamson's ether synthesis of the following is:
I. Me3CCH2Br
II. CH3CH2CH2Br
III. CH3=CHCH2Cl
IV. CH3CH2CH2ClAIIIgtIIgtIVgtIBIgtIIgtIVgtIIICIIgtIIIgtIVgtIDIgtIIIgtIIgtIV
Which compound in each of the following paris will react faster in SN2 reaction with HOo− ?
a.CH3Br or CH3I
b. (CH3)3Cl or CH3Cl
c. CH2=CHBr or CH2=CH−CH2Br
View SolutionWhich compound in each of the following pairs will react faster in SN2 reaction with –OH ?
(i) CH3BrorCH3I,(ii)(CH3)3CClorCH3Cl
View SolutionWhich compound in each of the following pairs will react faster in SN2 reaction with .–OH ?
(i) CH3BrorCH3I,(ii)(CH3)3CClorCH3Cl
View SolutionWhich compound in each of the following pairs will react faster in SN2 reaction with –OH ?
(i) CH3BrorCH3I,(ii)(CH3)3CClorCH3Cl
View SolutionArrange the folliwing compounds in increasing order of SN−2 reactivity.
a. I.m ClCH2CH=CHCH2CH3
II. CH3C(Cl)=CHCH2CH3
III. CH3CH=CHCH2CH2Cl
IV. CH3CH=CHCH2(Cl)CH3
b. I. CH3CH2Br
II. CH2=CHCH(Br)CH3
III. CH2=CHBr
IV. CH3CH(Br)CH3
C. I. (CH3)3Cl, II. C6H5C(CH3)2Cl
III. (CH3)2CHCl, IV. CH3CH2CH2Cl
View Solution

