Na and S are present in the third period of modern periodic table which is more metallic and why?Solution in Punjabi
Step by step video & image solution for Na and S are present in the third period of modern periodic table which is more metallic and why? by Chemistry experts to help you in doubts & scoring excellent marks in Class 10 exams.
Updated on:21/07/2023Similar Questions
Define Modern periodic law? Why many groups and periods are present in the long form of the periodic table?
View SolutionThe elements of the third period of the Periodic Table are given below:
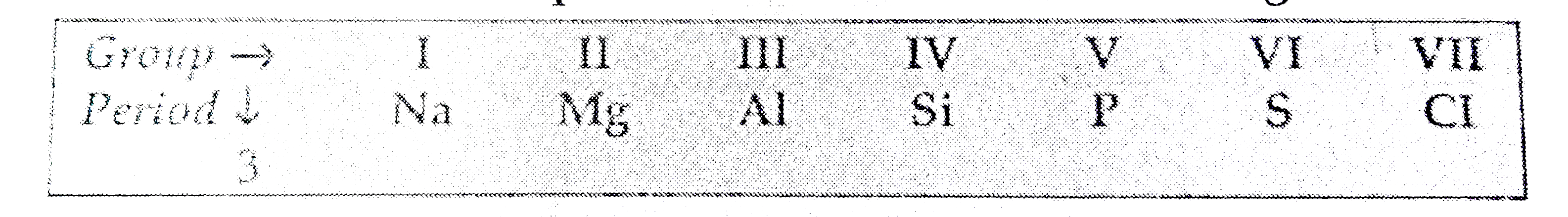
(a) Which atom is bigger, Na or Mg? Why?
(b) Identify the most (i) metallic and (ii) non-metallic element in Period 3.
(c ) Which is more non-metallic, S or Cl?
(d) Which has higher atomic mass, Al or Cl?
View Solutionआधुनिक आवर्त सारणी में कौनसे दो तत्व समान आवर्त तथा वर्ग में उपस्थित है :-
View SolutionWrite the number of elements present in first, third and fourth period in the modern periodic table.
View SolutionThe total number of elements in the second and third periods of the modern periodic table is ……….
View SolutionName or state with reference to the elements of the modern periodic table. The metals present in period 3 and the non-metals present in period 2.
View SolutionWhy the third period of Modern periodic table contains 8 elements and not 18 ?
View Solution