Explain SN2 mechanism taking an example of chloromethane.Solution in Kannada
Video Solution
Text Solution
When methyl bromide undergoes hydrolysis with aqueous potassiumhydroxide, methyl alcohol is formed.
CH3−Cl+KOH→CH3−OH+KCl
This mechanism involves only one step.
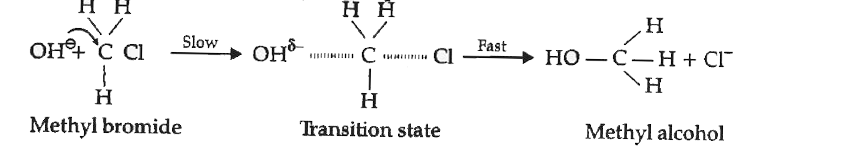
The nucleophile OH− attack from the rear side of the leaving group. A transition state with partial formation of C - OH bond and partial breaking of C - Br bond takes place simultaneously.
The rate of the reaction depends both on the concentration of nucleophile as well as alkyl halide. Hence, it is a second order reaction. In SN2 reaction, complete inversion of configruation takes place.
|
Similar Questions
Explain SN2 reaction mechanism of haloalkanes.
View SolutionExplain SN2 reaction mechanism of haloalkanes.
View SolutionExplain the mechanism of SN1 reaction taking 2-bromo-2-methyl propane (t-butyl bromide)
View SolutionExplain the mechanism of SN1andSN2 reactions with examples.
View SolutionExplain with example SN1 mechanism.
View SolutionExplain SN−2 reaction mechanism ?
View Solutioni) Explain SN2 mechanism taking an example of chloromethane.
ii) Write the general equation for the reaction of primary alcohol with SOCl2.
View Solution

