Write a short note on:
Structure of the Modern periodic table Solution in Marathi
Video Solution
Text Solution
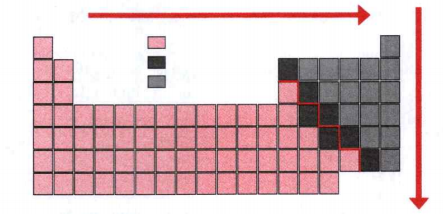
i. The modern periodic table consists of seven horizontal rows called the periods and eighteen vertical columns called the groups.
ii. The arrangement of the periods and groups results in the formation of boxes, where each box corresponds to the position of an element.
iii. In addition to these seven rows, lanthanide and actinide series are shown separately at the bottom of the periodic table. The first row is lanthanide series and the second row is actinide series.
iv. The entire periodic table is divided into four block: s-block, p-block, d-block and f-blolck.
v. s-block: contains group 1 and 2 elements
p-block: contains group 13 to 18 elements
d-block: contains group 3 to 12 elements (transition elements)
f-block: contains lanthanide and actinide series
vi. A zig-zag line is drawn in the p-block of the periodic table. Metals lie on the left side while nonmetals lie on the right side of this line. Metalloids lie along the border of this line.
|
Similar Questions
Write a short note on Modern English .
View SolutionWrite a short note on: Structure of the Modern periodic table
View SolutionWrite a short note on: Position of isotpes in the Mendeleev's and the Modern periodic table
View SolutionWrite the answers of the question with refernce to the structure of the periodic table.
 which points are considered for the arrangement of the Modern period table?
which points are considered for the arrangement of the Modern period table?
View SolutionWrite short notes on class and period.
View SolutionStructure of the modern periodic table .
View SolutionWrite the modern periodic table.
View Solutionटिपा लिहा.
आधुनिक आवर्त सारणाची रचना
View Solution
