In a reaction, between A and B, the initial rate of reaction (r0) was measured for different initial concentrations of A and B as given below :

What is the order of reaction with respect to A and B ?
r0=k[A]α[B]β
(r0)1=k×5.07×10−5=(0.20)α(0.30)β …(i)
(r0)2=k×5.07×10−5=(0.20)α(0.10)β …(ii)
(r0)3=k×1.43×10−4=(0.40)α(0.05)β ...(iii)
Dividing (i) by (ii)
(r0)1/(r0)2=1=(0.30/0.10)β=(3)β
∴β=0
Dividing (iii) by (ii)
(r0)3/(r0)2=7.165.07=(0.40/0.20)α(0.05/0.10)β
or 1.412=2α(12)β=2α [∵β=0]
or log1.412=αlog2
or α=0.1523/0.3010=0.5
Thus, order w.r.t. A = 0.5, order w.r.t. B = 0.
Similar Questions
In a reaction between A and B, the initial rate of reaction was measured for different initial concentrations of A and B as given below:
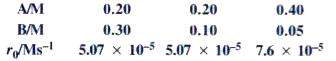
What is the order of reaction with respect to A and B ?
View SolutionIn a reaction between A and B, the initial rate of reaction was measured for different initial concentrations of A and B as given below:
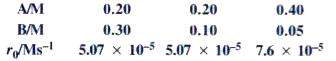
What is the order of reaction with respect to A and B ?
View Solution
Knowledge Check
In a reaction between A and B, the initial rate of reaction r0 was measured for different initial concentrations of A and B as given below :

The order of the reaction with respect to A isA1.5B0.5C1D2In a reaction between A and B, the initial rate of reaction was measured for different initial concentration A and B as:
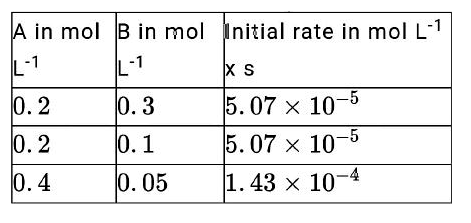
Find the order of reaction with respect to A and B.A1,0B1,1C1,5,0D1,5,1For a reaction between A and B, the initial rate of reaction is measured for various initial concentrations of A and B . The data provided are :
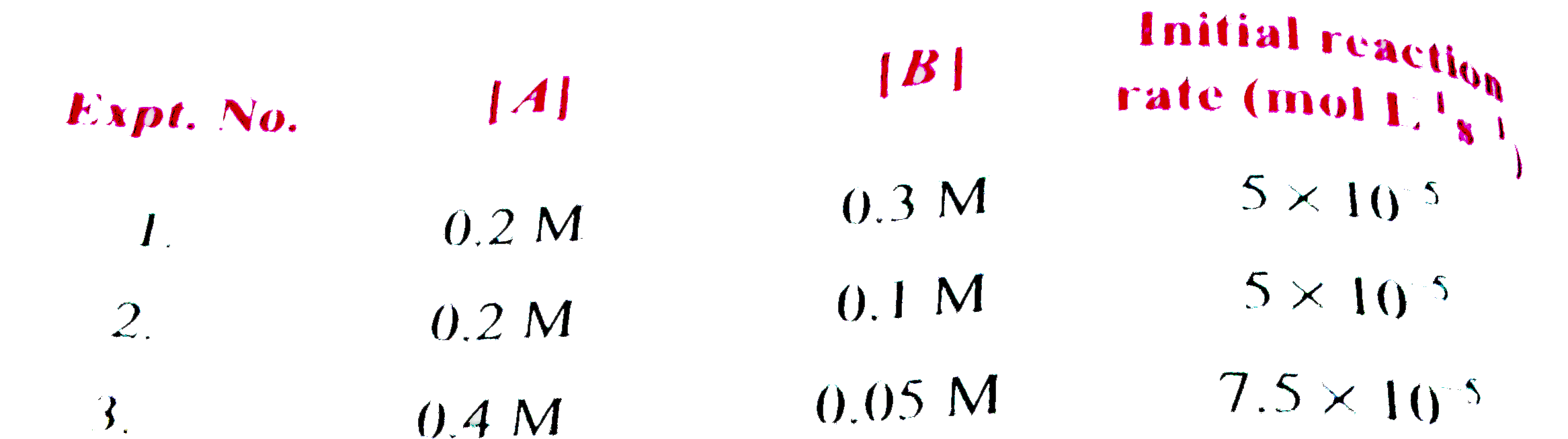
The overall order of the reaction is :Aone(1)Btwo (2)Ctwo and a half (2.5)Dbetween 0 and 1
In a reaction between A and B, the initial rate of reaction was measured for different initial concentrations of A and B as given below:
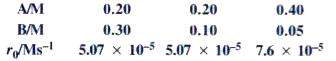
What is the order of reaction with respect to A and B ?
View SolutionIn a reaction between A and B, the initial rate of reaction was measured for different initial concentration of A and B as given ahead:

what is the order of reaction with respect to A and B?
View SolutionConsider the reaction, A+B→C+D.
The initial rate for different initial concentrations of the reactants are given below :
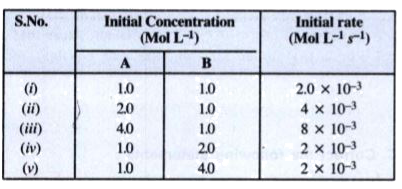
What are the orders with respect to A and B ?
View SolutionConsider the reaction, A+B→C+D.
The initial rate for different initial concentrations of the reactants are given below :
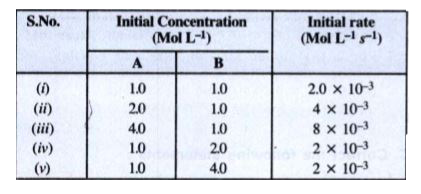
Calculate the rate constant.
View SolutionFor a reaction between A and B, the initial rate of reaction is measured for various initial concentrations of A and B. The data provided are

The overall order of the reaction is
View Solution

