For a general reaction A→B, plot of concentration of A vs time is given in the figure below. Answer the following questions on the basis of this graph.
(i) What is the order of the reaction ?
(ii) What is the slope of the curve ?
(iii) What are the units of the rate constant ?

(i) For a zero order reaction, a plot of [A] with t gives the above type of graph. Hence this is a zero order reaction.
(ii) Slope of the curve = - k
(iii) Units of rate constant for zero order reaction are mol L−1s−1.
Topper's Solved these Questions
- CHEMICAL KINETICS Book:U-LIKE SERIESChapter:CHEMICAL KINETICS Exercise:LONG ANSWER QUESTIONS-I
Explore 26 Videos
- CHEMICAL KINETICS Book:U-LIKE SERIESChapter:CHEMICAL KINETICS Exercise:LONG ANSWER QUESTIONS-II
Explore 13 Videos
- CHEMICAL KINETICS Book:U-LIKE SERIESChapter:CHEMICAL KINETICS Exercise:VERY SHORT ANSWER QUESTIONS
Explore 47 Videos
- CBSE EXAMINATION PAPER 2020 (CODE No.56/4/1) Book:U-LIKE SERIESChapter:CBSE EXAMINATION PAPER 2020 (CODE No.56/4/1) Exercise:SECTION -D
Explore 3 Videos
- CHEMISTRY IN EVERYDAY LIFEBook:U-LIKE SERIESChapter:CHEMISTRY IN EVERYDAY LIFEExercise:LONG ANSWER QUESTIONS - I
Explore 21 Videos
Similar Questions
For a general reaction A→B, plot of concentration of A vs time is given in figure. Answer the following questions on the basis of this graph.
(i) what is the order of the reaction?
(ii) What is the slope of the curve ?
(iii) What are the units of rate constant?
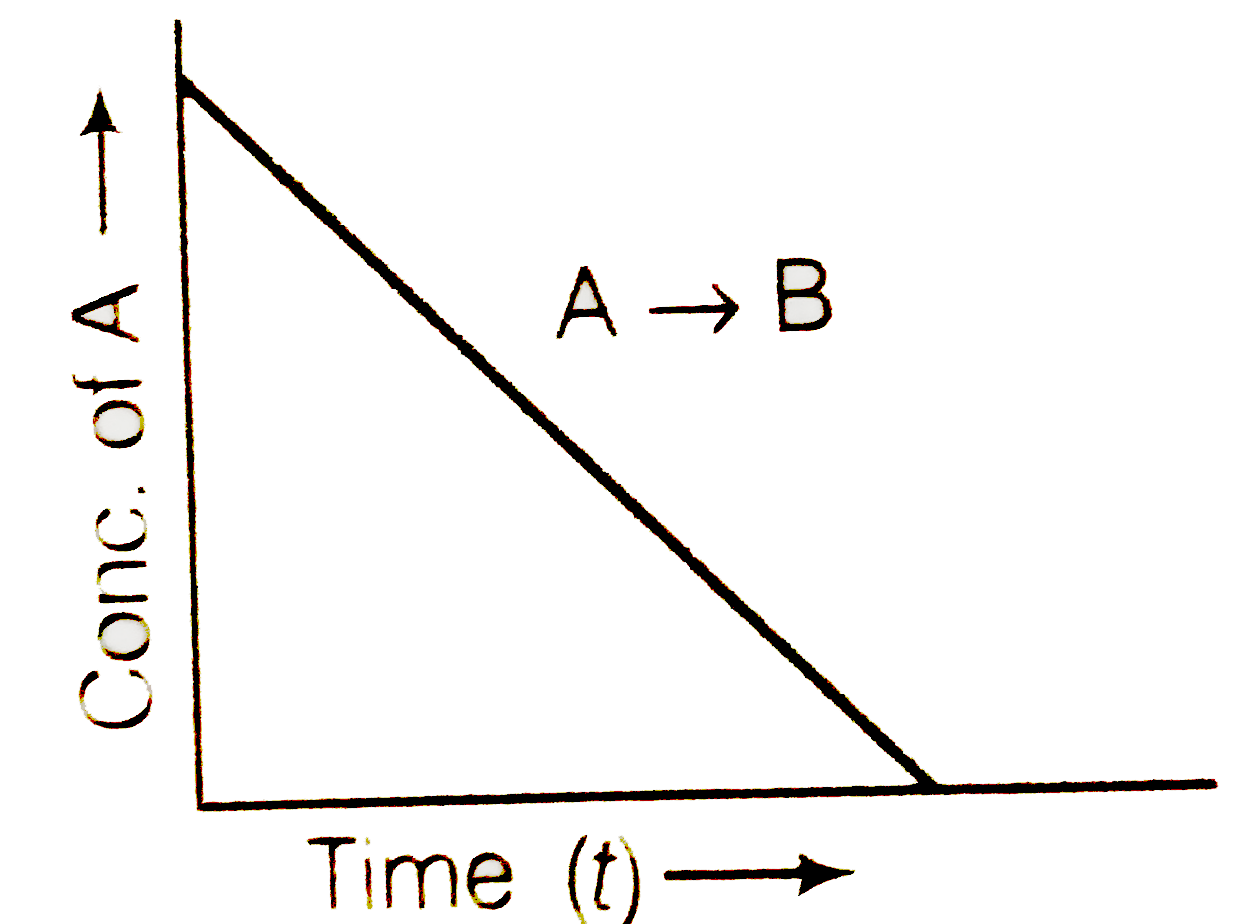
View SolutionFor a general reaction A→B, plot of concentration of A vs time is given in figure. Answer the following questions on the basis of this graph.
(i) what is the order of the reaction?
(ii) What is the slope of the curve ?
(iii) What are the units of rate constant?
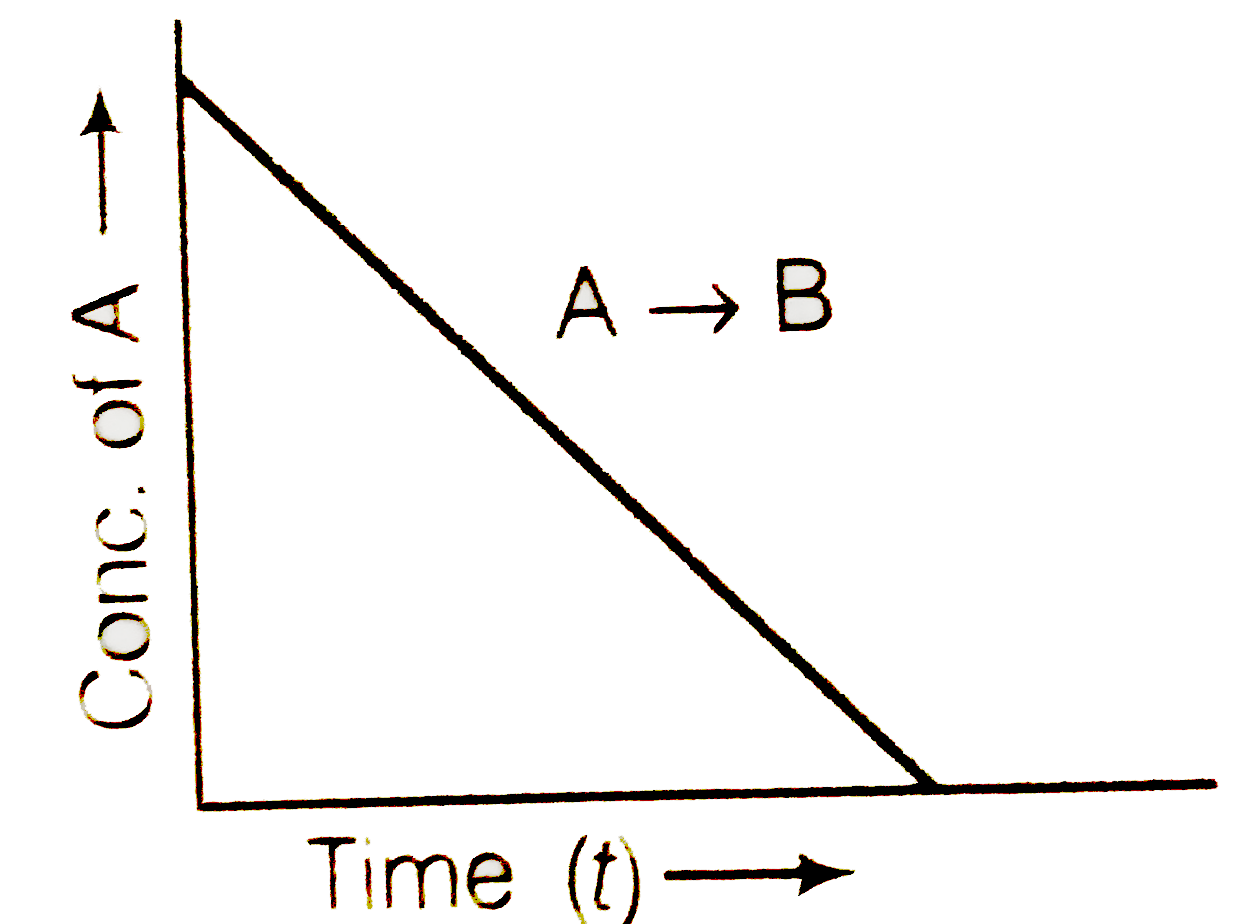
View Solution
Knowledge Check
What are the units for the rate constant of a zero-order reaction?
AtimeBtime−1CM.timeDM.time−1
For a general reaction A→B. plot of concentrating of A vs time is given in fig.
Answer the following questions on the basis of this graph.
a) What is the order of the reaction?
b) What is the slope of the curve?
c) What are the units of rate constant?

View SolutionFor a general reaction X→Y, the plot of conc. Of X vx time is given in the figure. What is the order of the reaction and what are the units of rate constant?
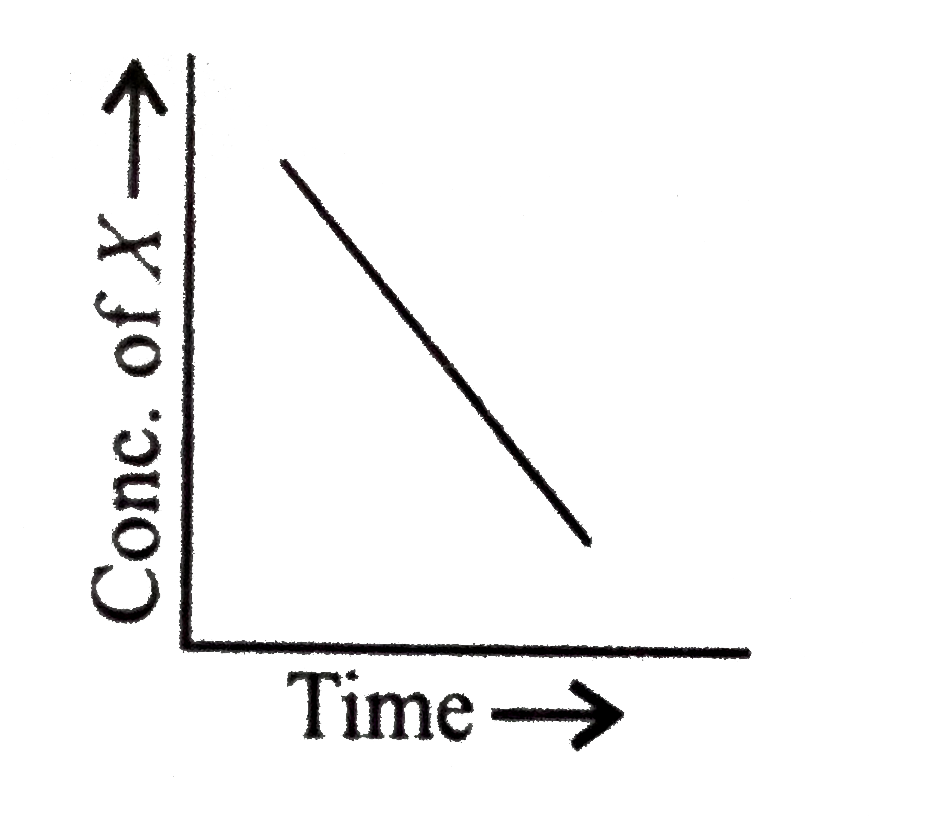
View SolutionFor a general reaction X→Y, the plot of conc. Of X vs Time is given in the figure. What is the order of the reaction and what are the units of rate constant?

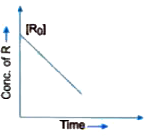
View SolutionVisha plotted a graph between concentration of R and time for a reaction R→P. On the basis of this graph, answer the following question :
(i) Predict the order of reaction.
(ii) What does the slope of the line indicate ?
(iii) What are the units of rate constant ?
[Give :log2=0.3010,log3=0.4771,log4=0.6021]
View SolutionWhat are the units of rate constant for zero order reactions?
View SolutionWhat are the units of rate constant for third order reaction?
View SolutionWhat are the units of rate constant for a first order reaction?
View Solution

