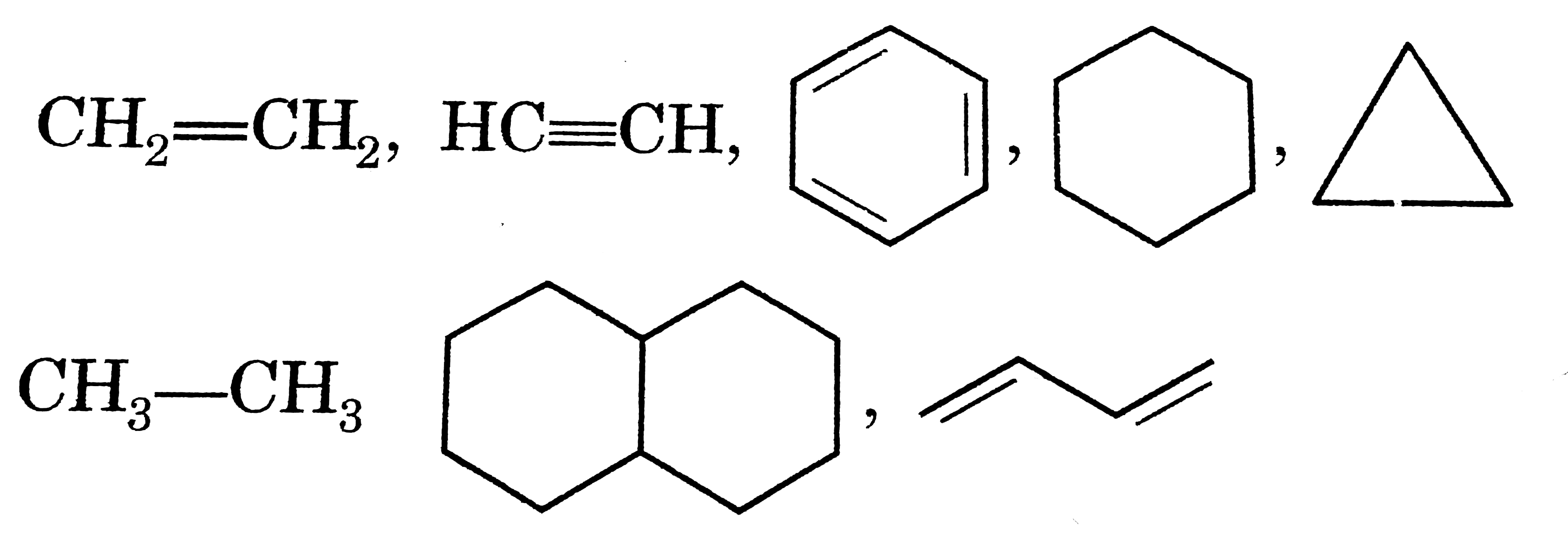
Which of the following hydrocarbon can decolourise bromine water?
C4H6
C4H8
C3H6
all these
The correct Answer is:D
To determine which hydrocarbons can decolourise bromine water, we need to identify the unsaturated hydrocarbons among the given options. Unsaturated hydrocarbons contain either double bonds (alkenes) or triple bonds (alkynes).
1. Understand the Concept of Unsaturation:
- Unsaturated hydrocarbons are those that contain at least one double bond (alkenes) or triple bond (alkynes). These bonds can react with bromine, leading to the decolourisation of bromine water.
2. Examine the Given Hydrocarbons:
- Let's analyze the hydrocarbons provided:
- C4H6: This hydrocarbon can be represented as having a triple bond (alkyne), which makes it unsaturated.
- C4H8: This hydrocarbon can have a double bond (alkene), indicating it is also unsaturated.
- C3H6: This hydrocarbon can also have a double bond (alkene), confirming it as unsaturated.
3. Conclusion:
- Since all three hydrocarbons (C4H6, C4H8, and C3H6) are unsaturated, they can all decolourise bromine water.
4. Final Answer:
- Therefore, all of the given hydrocarbons can decolourise bromine water.
- Unsaturated hydrocarbons are those that contain at least one double bond (alkenes) or triple bond (alkynes). These bonds can react with bromine, leading to the decolourisation of bromine water.
- Let's analyze the hydrocarbons provided:
- C4H6: This hydrocarbon can be represented as having a triple bond (alkyne), which makes it unsaturated.
- C4H8: This hydrocarbon can have a double bond (alkene), indicating it is also unsaturated.
- C3H6: This hydrocarbon can also have a double bond (alkene), confirming it as unsaturated.
- Since all three hydrocarbons (C4H6, C4H8, and C3H6) are unsaturated, they can all decolourise bromine water.
- Therefore, all of the given hydrocarbons can decolourise bromine water.
Similar Questions
Knowledge Check
Which of the following hydrocarbons does not decolourise bromine water?
AC10H22BC6H12CC10H18DC10H20Which of the following organic acid decolourises bromine water as well as forms anhydride ?
AHOOC−COOHBHOOC−CH2−COOHCH−C−COOH∣∣H−C−COOHDH−C−COOH∣∣HOOC−C−HThe following hydrocarbon can exhibit.
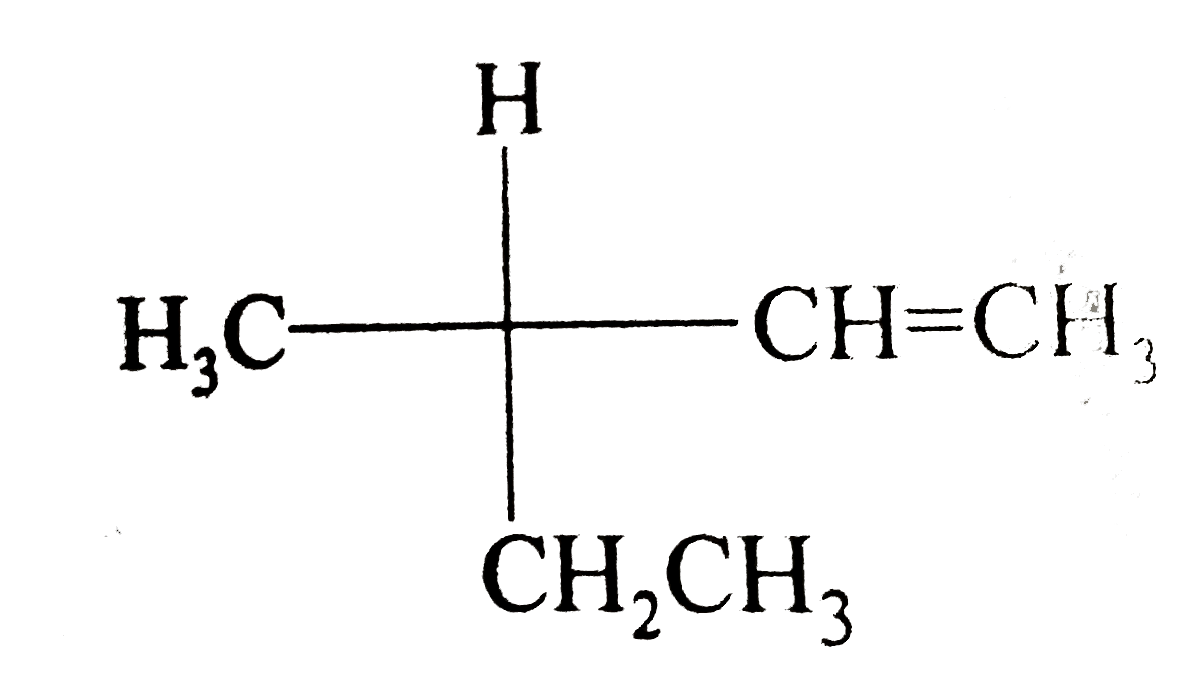 AGeometrical isomersBoptical isomerismCBoth geometrical and optical isomerismDTautomerism
AGeometrical isomersBoptical isomerismCBoth geometrical and optical isomerismDTautomerism
Which of the following can decolourise bromine water solutions ?
View SolutionWhich of the following compound does not decolourise bromine-water solution ?
View SolutionWhich of the following compound decolourises bromine water also reacts with PCl5 to give white fumes of HCl?
View SolutionWhich of the following does not decolourise iodine?
View SolutionWhich of the following can decolourless bromine wate solution?
View Solution