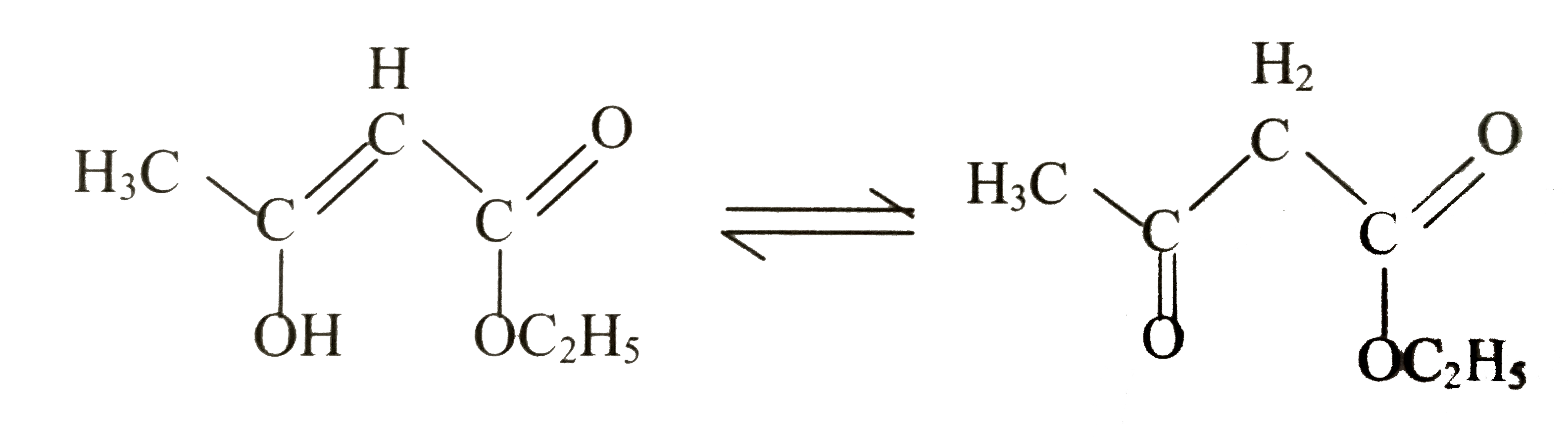S1 : enol form of acetone < 0.1% and enol form of acetylacetone is 15%.
S2 enol form of acetyl acetone is stabilized by intramolecular H-Bonding which is not possible in acetone enol form.
Both S1&S2 are correct
S1 is correct & S2 is wrong
S2 is correct & S1 is wrong
Both S1&S2 are wrong
Step by step video & image solution for S_1 : enol form of acetone lt 0.1% and enol form of acetylacetone is 15%. S_2 enol form of acetyl acetone is stabilized by intramolecular H-Bonding which is not possible in acetone enol form. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Assertion A: Enol form of acetone [CH3COCH3] exists in <0.1% quantity. However, the enol form of acetyl acetone [CH3COCH2OCH3] exists in approximately 15% quantity
Reason R: Enol form of acetyl acetone is stabilized by intramolecular hydrogen bonding which is not possible in enol form of acetone.
Choose the correct statement :
View SolutionThe enolic form of ethyl acetoacetate as below has
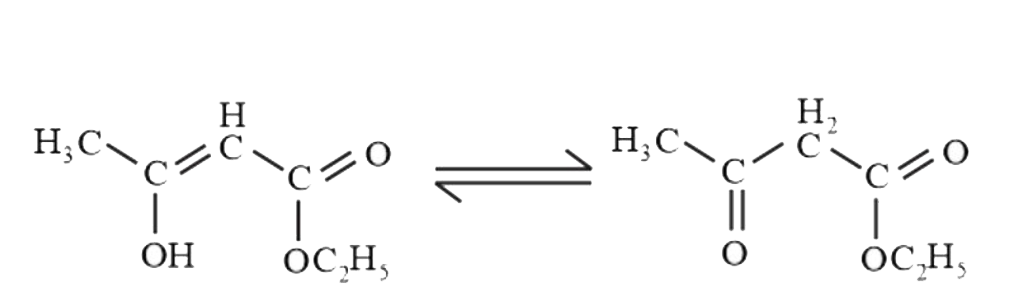
View Solution
Knowledge Check
Enolic form of acetyl acetone is stablised due to :
Aresonance as a result of conjugationBintramolecular hydrogen bondingCvan der wall forceDdipole-dipole repulsionThe enolic form of acetone contains
Anine σ -bonds, one π -bond and two lone pairsBeight σ-bonds, two π -bonds and two lone pairsCnine σ -bonds, one π -bond and one lone pairsDnine σ -bonds, two π -bond and one lone pairsThe enolic form of acetone contains
A9 sigma bonds, 1 pi bond and 2 lone pairsB8 sigma bonds, 2 pi bonds and 2 lone pairsC10 sigma bonds, 1 pi bond and 1 lone pairD9 sigma bonds, 2 pi bonds and 1 lone pair