The enthalpy changes for the reaction C2H4(g)+H2(g)→C2H6(g) is -620J When 100ml of ethylene and 100ml of H2reacts at 1 bar pressure. Calculate the pressure volume type of work and ΔU for the reactionSolution in Marathi
Step by step video & image solution for The enthalpy changes for the reaction C_2H_4(g) + H_2(g) rarr C_2H_6(g) is -620J When 100ml of ethylene and 100ml of H_2reacts at 1 bar pressure. Calculate the pressure volume type of work and DeltaU for the reaction by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Kp = 0.04 atm at 899 K for the equilibrium shown below, what is the equilibrium pressure of C2H6 when it is placed in a flask at 4.0 atm pressure and allowed to come to equilibrium ? C2H6(g)⇔C2H4(g)+H2(g)
View SolutionFor the gas phase reaction, C_2H_4(g) + H_2(g) iff C_2H_6: DeltaH = 32.7 kcal carried out in a vessel, the equilibrium concentration of C_2H_4 can be increased by
View SolutionThe enthalpy change of the reaction C3H8(g)+H2(g)→C2H6(g)+CH4(g), at 25∘, will be Given that: heat of combustion values under standard condition
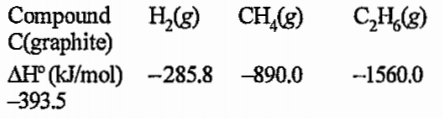 (The standard heat of formation of C3H8(g) is
-103.8kJ/mol)
(The standard heat of formation of C3H8(g) is
-103.8kJ/mol)
View SolutionEnthalpy of formation of ammonia is - '46.0 kJ mol^(-1)'. The enthalpy change for the reaction: '2 NH_3(g) rarr N_2(g)+3 H_2(g)'
View SolutionKp=0.04 at 899 K for the equilibrium shoen below.What is the equilibrium concentration of C2H6 when it is placed in a flask at 4.0 atm pressure and allowed to come to equilibrium?C2H6(g)⇔C2H4(g)+H2(g)
View SolutionClassify the following reactions as
C2H4(g)+H2→C2H6(g)
View Solution200 mL ethylene gas and 150 mL of HCl gas were allowed to react at 1 bar pressure according to the reaction
C2H4(g)+HCl(g)→C2H5Cl(g)
Calculate the PV work in joules.
View SolutionCalculate ΔU at 298 K for the reaction,
C2H4(g)+HCl(g)→C2H5Cl(g),ΔH=−72.3kJ
How much PV work is done?
View Solution

