Consider a P-V diagram in which the path followed by one mole of perfect gas in a cylindrical container as shown in Fig 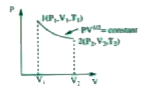
a) Find the work done when the gas is taken from state 1 to state 2.
b) What is the ratio of temperature T1/T2, if V2=2V1 ?
c) Given the internal energy for one mole of gas at temperature T is (3/2) RT, find the heat supplied to the gas when it is taken from state 1 to 2, with V2=2V1 .
a) Work done by the gas (LetPV1/2=A)
ΔW=∫V2V1pdv=A∫V2V1dV√V=A[√V1/2]V2V1=2A(√V2−√V1)=2P1V1/21[V1/22−V1/21]
b) Since T=pV/nR=AnR.√V
Thus T2T1=√V2V1=√2
c) Then, the change in internal energy
ΔU=U2−U1=32R(T2_T1)=32RT1(√2−1)
ΔW=2A√V1(√2−1)=2RT1(√2−1)
ΔQ=(7/2)RT1(√2−1)
Topper's Solved these Questions
- THERMODYNAMICSBOOK - AAKASH SERIESCHAPTER - THERMODYNAMICSEXERCISE - EXERCISE - 1A
135 Videos
- THERMODYNAMICSBOOK - AAKASH SERIESCHAPTER - THERMODYNAMICSEXERCISE - EXERCISE - 1B
32 Videos
- THERMODYNAMICSBOOK - AAKASH SERIESCHAPTER - THERMODYNAMICSEXERCISE - QUESTIONS FOR DESCRIPTIVE ANSWERS
7 Videos
- THERMAL PROPERTIES OF MATTERBOOK - AAKASH SERIESCHAPTER - THERMAL PROPERTIES OF MATTEREXERCISE - ADDITIONAL PRACTICE EXERCISE (LEVEL - II) PRACTICE SHEET (ADVANCED) Integer/Subjective Type Questions
2 Videos
- THERMOMETRY BOOK - AAKASH SERIESCHAPTER - THERMOMETRY EXERCISE - Numerical Exercise (LEVEL- 1)
11 Videos
Similar Questions
Consider a P−V diagram in which the path followed by one mole of perfect gas in a cyclinderical container is shown in (figure)
(a) Find the work done when the gas is taken from state 1 to state 2.
(b) What is the ratio of temperatures T1/T2,ifV2=2V1?
(c ) Given the internal energy for one mole of gas at temperature Tis(3/2)RT, find the heat supplied to the gas when it is taken from state 1 to 2, with V2=2V1.
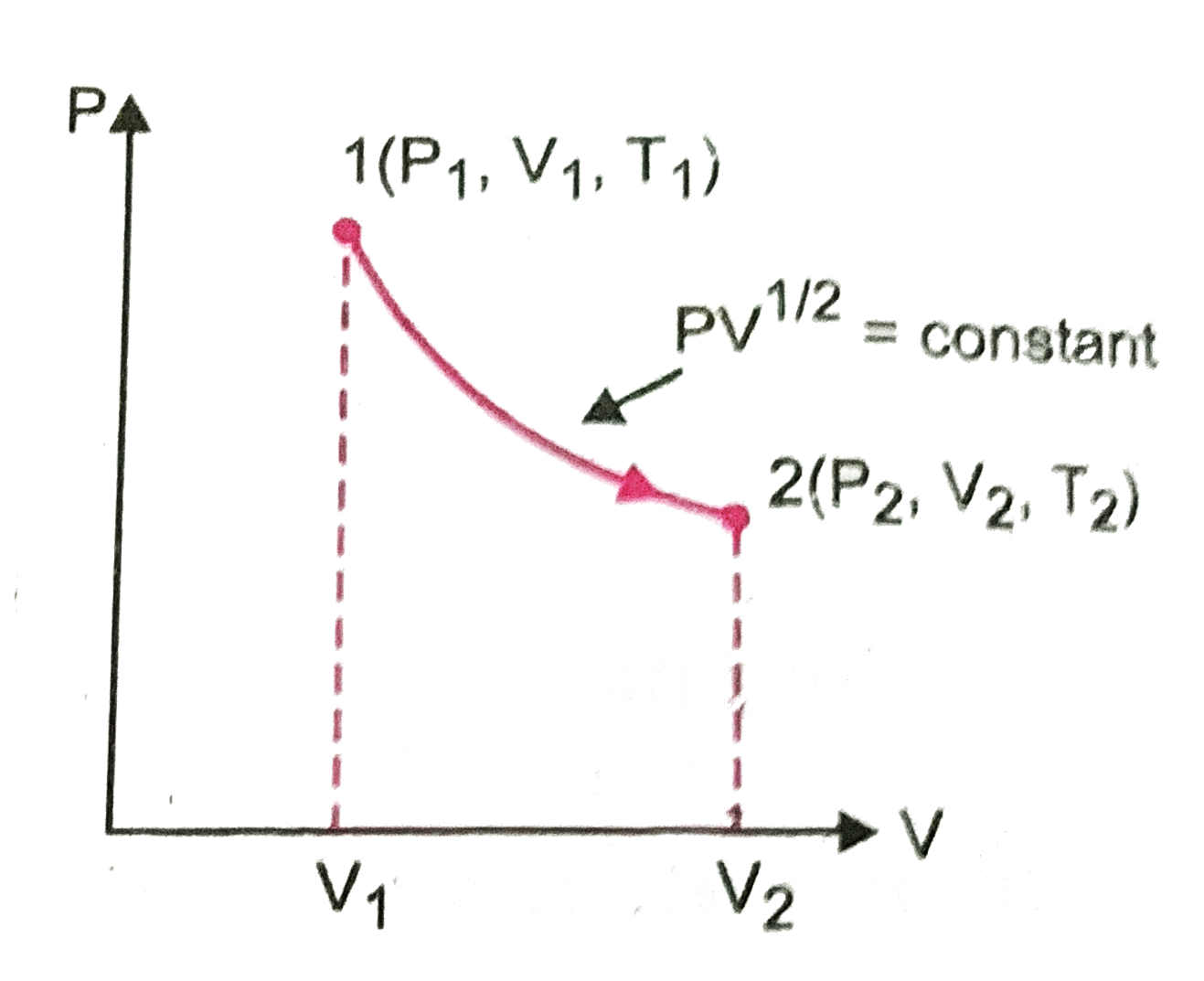
View SolutionConsider a P−V diagram in which the path followed by one mole of perfect gas in a cyclinderical container is shown in (figure)
(a) Find the work done when the gas is taken from state 1 to state 2.
(b) What is the ratio of temperatures T1/T2,ifV2=2V1?
(c ) Given the internal energy for one mole of gas at temperature Tis(3/2)RT, find the heat supplied to the gas when it is taken from state 1 to 2, with V2=2V1.
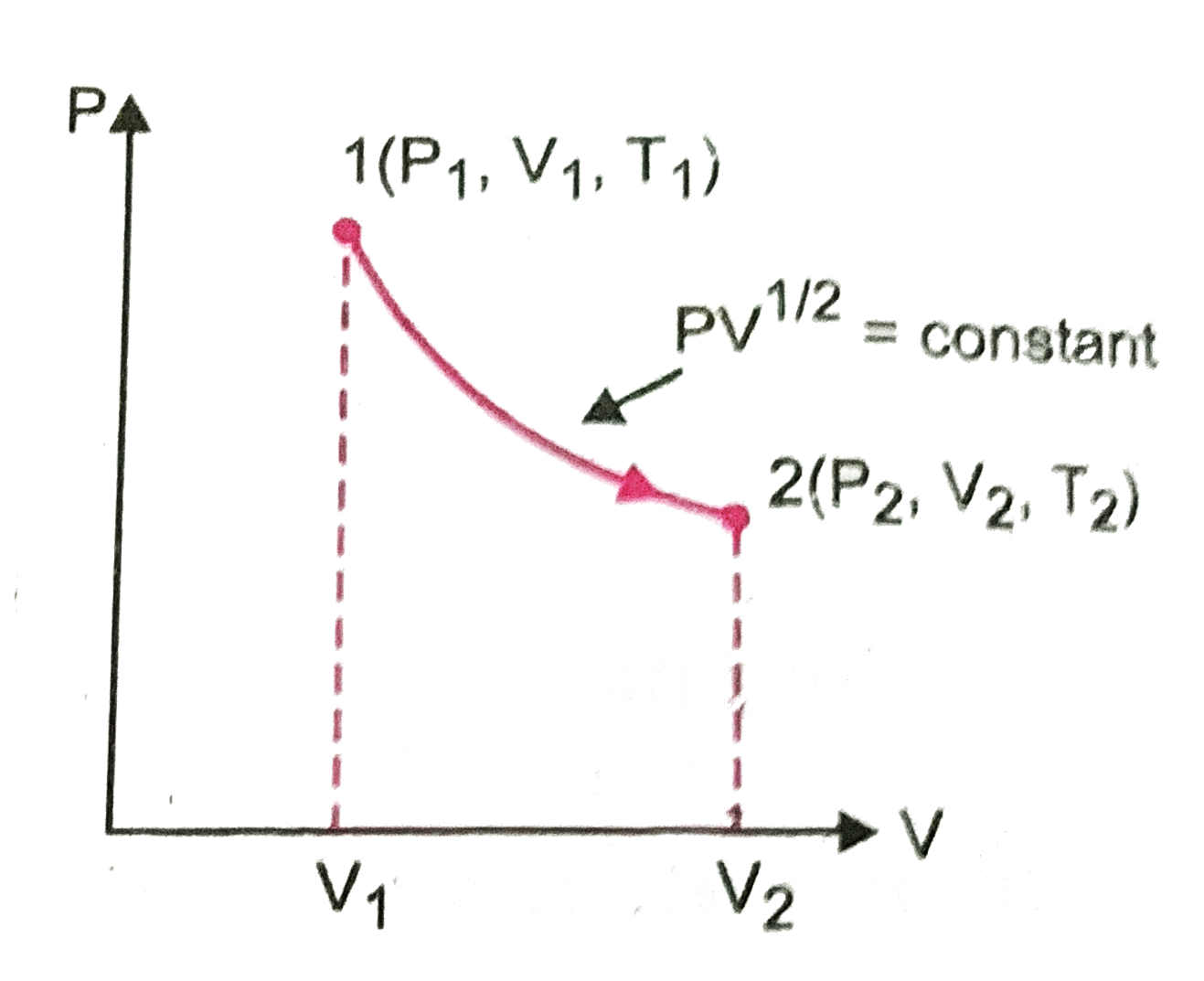
View Solution
Knowledge Check
The P-V diagram of path followed by one mole of perfect gas in a cylindrical container is shown in figure, the work done when the gas is taken from state A to state B is
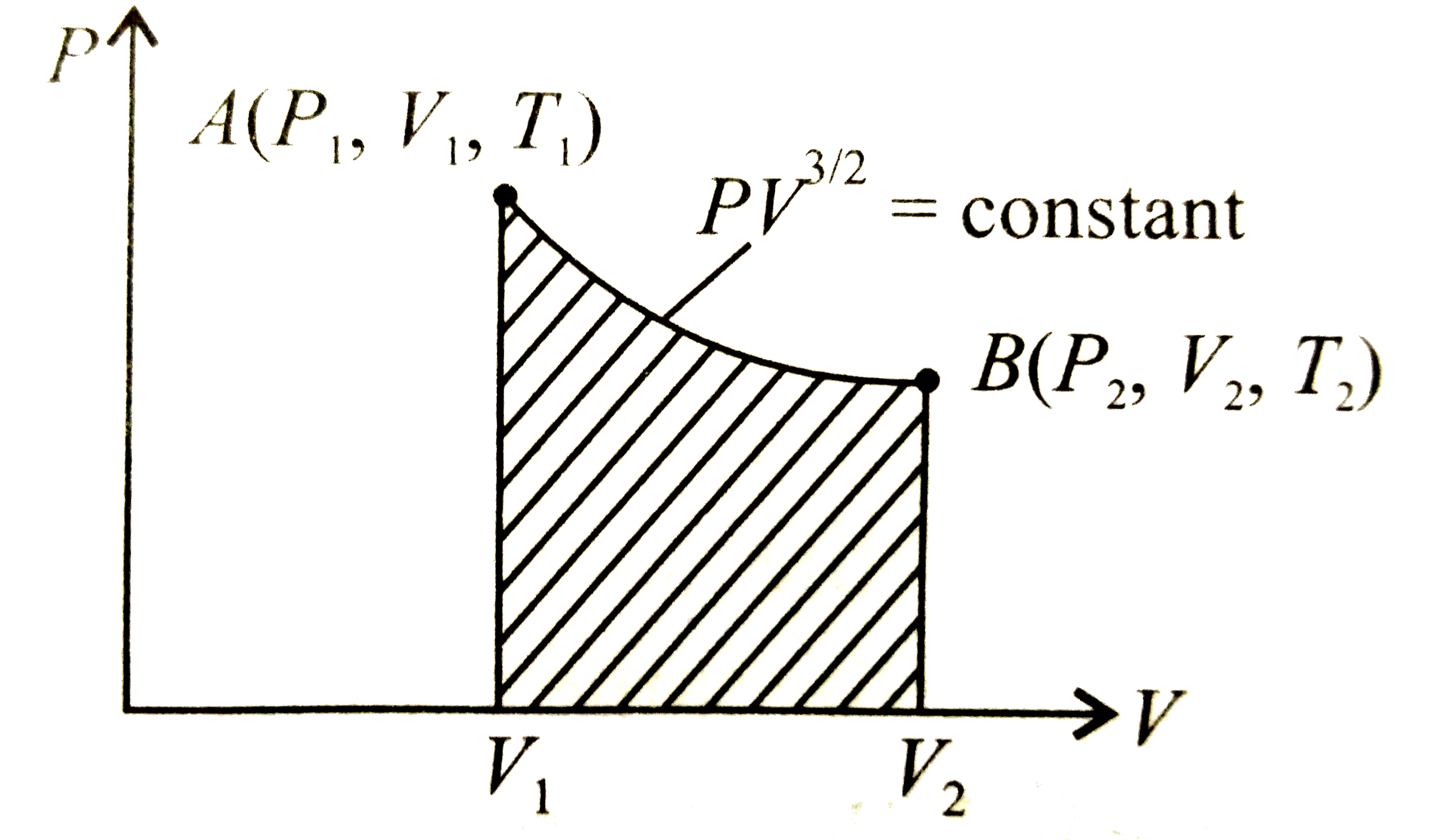 AnRTInV2V1BnRTInV1V2C2nRT((V1)−12−((V2)−12)D2nRTInV1V2
AnRTInV2V1BnRTInV1V2C2nRT((V1)−12−((V2)−12)D2nRTInV1V2The P-V diagram of path followed by one mole of perfect gas in a cylindrical container is hsown in figure, the work done when the gas is taken from state A to state B is
 AnRTInV2V1BnRTInV1V2C2nRTInV2V1D2nRTInV1V2
AnRTInV2V1BnRTInV1V2C2nRTInV2V1D2nRTInV1V21 mole of an ideal gas in a cylindrical container have the P-V diagram as shown in figure.If V2=4V1 then the ratio of temperatures T1T2 will be
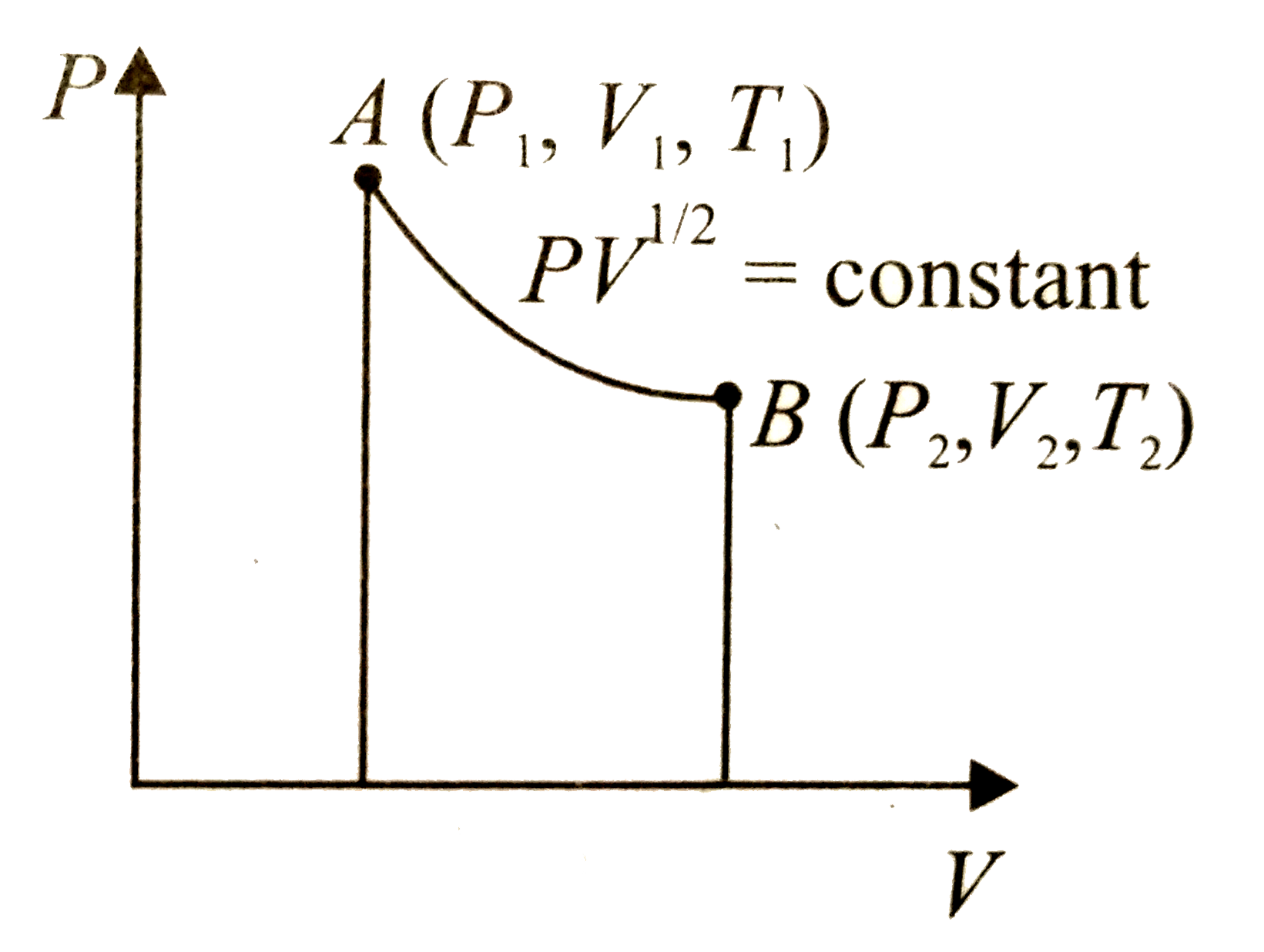 A12B14C32D34
A12B14C32D34
n moles of an ideal gas is taken through a four step cyclic process as shown in the diagram. Calculate work done by the gas in a cycle in terms of temperatures T1,T2,T3 and T4
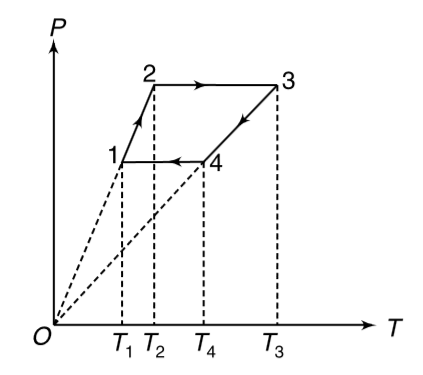
View SolutionInternal energy of n moles of helium at temperature T1K is equal to the internal energy of 2n moles of oxygen gas at temperature T2K then the value of T1T2will be
View SolutionInternal energy of n moles of helium at temperature T1K is equal to the internal energy of 2n moles of oxygen gas at temperature T2K then the value of T1T2will be
View Solution1 mole of an ideal gas in a cylindrical container have the P-V diagram as shown in figure.If V2=4V1 then the ratio of temperatures T1T2 will be
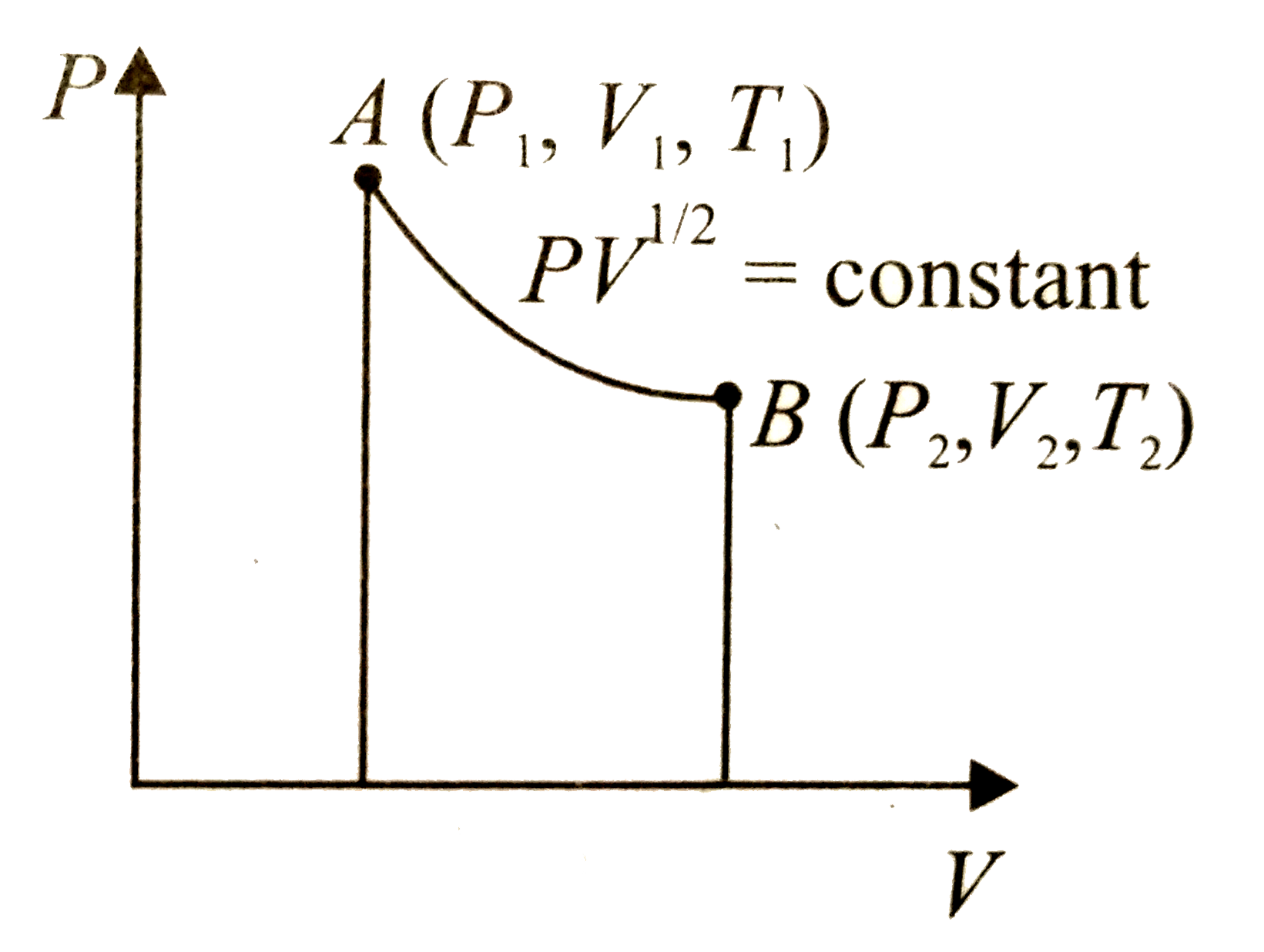
View SolutionOne mole of an ideal gas at temperature T1 expands according to the law (P/V) = constant. Find the work done when the final temperature becomes T2.
View Solution

