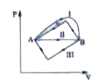
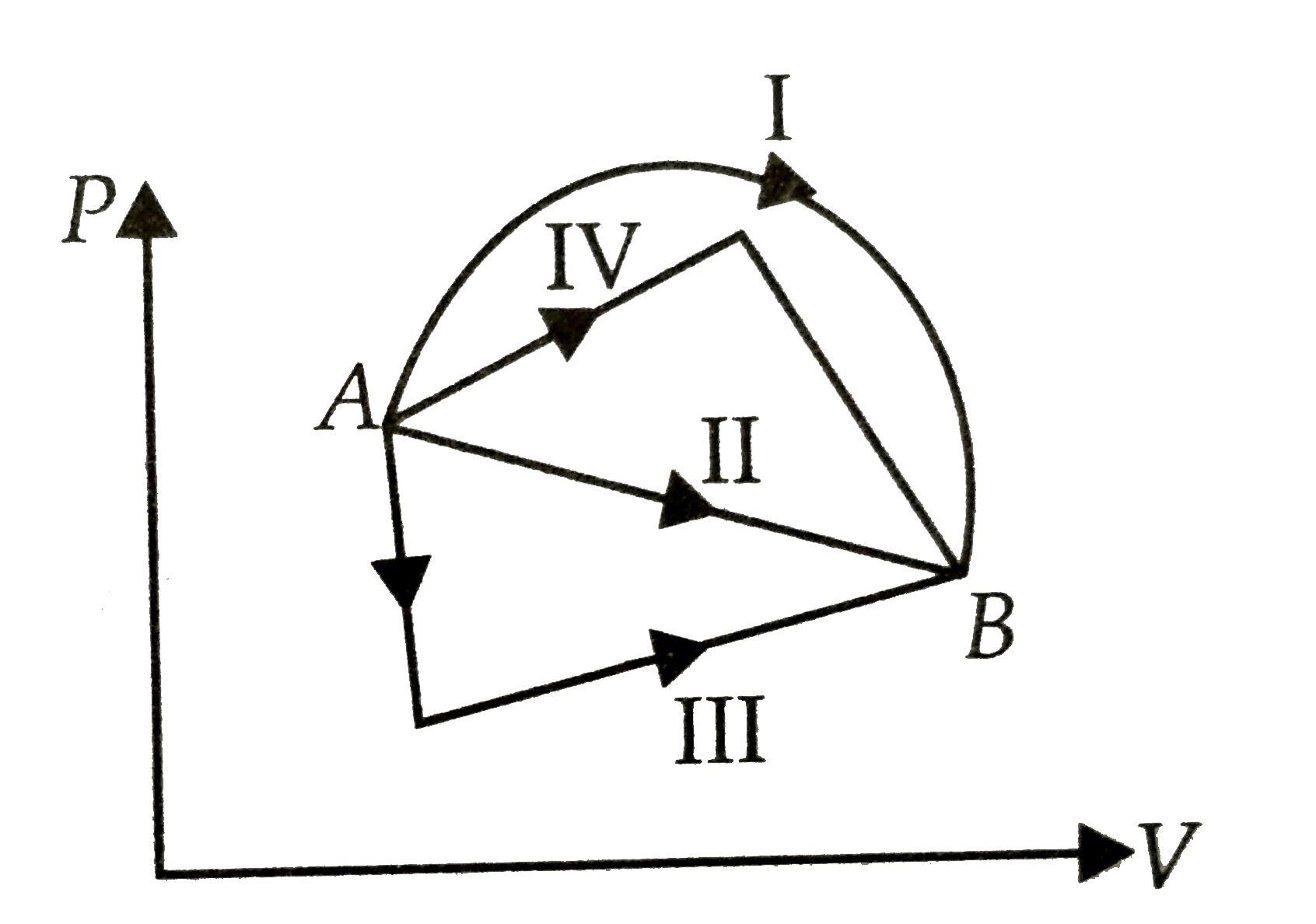
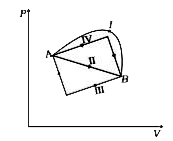
Figure 12.5 shows the P-V diagram of an ideal gas undergoing a change of state from A and B. Four different parts I,II,III and IV as shown in the figure may lead to the same change of state.
a) Change in internal energy is same in IV and III cases, but not I and II
b) Change in internal energy is same in all the four cases
c) Work done is maximum in case I
d) Work done is minimum in case II
 Solution in Telugu
Solution in Telugu
Only a, b are correct
only b, d are correct
only c, d are correct
only b and c are correct
The correct Answer is:D
Step by step video, text & image solution for Figure 12.5 shows the P-V diagram of an ideal gas undergoing a change of state from A and B. Four different parts I,II,III and IV as shown in the figure may lead to the same change of state. a) Change in internal energy is same in IV and III cases, but not I and II b) Change in internal energy is same in all the four cases c) Work done is maximum in case I d) Work done is minimum in case II by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
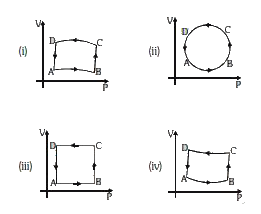
चित्र (i) से (iv) में दाब में परिवर्तन के साथ आयतन परिवर्तनदर्शाया गया है। एक गैस को पथ ABCDA के अनुदिश ले जाया जाता है। गैस की आन्तरिक ऊर्जा में परिवर्तन है :
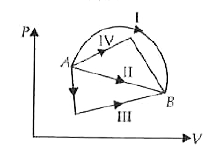
View SolutionAn ideal gas undergoing a change of state from A to B through four different paths I, II,III and IV as shown in the P-V diagram that lead to the same change of state tyhen the change in internal energy is
View SolutionP-V diagram of an ideal gas for a process ABC is as shown in the figure.
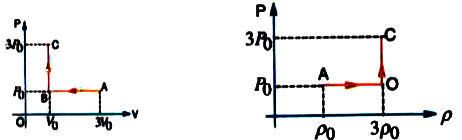
Change in internal energy of the gas during the process ABC
View Solutionचित्र 12.5 में A से B तक किसी आदर्श गैस की अवस्था परिवर्तन का P-V आरेख दर्शाया गया है। इसके चार विभिन्न भाग I, II, III तथा IV आरेख में दिए अनुसार समान अवस्था परिवर्तन की ओर संकेत करते हैं।
View Solutionएक आदर्श गैस में चित्रानुसार P- V की तरह, चार विभिन्न पथों I, II, III व IV के माध्यम से A से B में अवस्था परिवर्तन होता है। इसके परिणामस्वरूप, अवस्थाओं में समान परिवर्तन होता है, तब आंतरिक ऊर्जा में परिवर्तन है-
View SolutionStatement I: When an ideal gas is taken from a given thermodynamics state A to another given thermodynamic state B by any polytropic process, the change in the internal energy of the system will be the same in all processes.
Statement II: Internal energy of the ideal gas depends only upon its absolute temperature.
View Solution0.5 moles of an ideal monoatomic gas undergoes a thermodynamic process from A to B as shown in figure. The change in internal energy of the gas during the process from A to B is
View Solution
