Heat energy absorbed by a system in going through a cyclic process shown in figure is
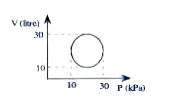 Solution in Telugu
Solution in Telugu
107πJ
104πJ
102πJ
10−3πJ
The correct Answer is:C
Step by step video, text & image solution for Heat energy absorbed by a system in going through a cyclic process shown in figure is by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- THERMODYNAMICSBook:AAKASH SERIESChapter:THERMODYNAMICSExercise:EXERCISE - 3
Explore 33 Videos
- THERMODYNAMICSBook:AAKASH SERIESChapter:THERMODYNAMICSExercise:EXERCISE - 1B
Explore 32 Videos
- THERMAL PROPERTIES OF MATTERBook:AAKASH SERIESChapter:THERMAL PROPERTIES OF MATTERExercise:ADDITIONAL PRACTICE EXERCISE (LEVEL - II) PRACTICE SHEET (ADVANCED) Integer/Subjective Type Questions
Explore 2 Videos
- THERMOMETRY Book:AAKASH SERIESChapter:THERMOMETRY Exercise:Numerical Exercise (LEVEL- 1)
Explore 11 Videos
Similar Questions
A bullet of mass 10×10−3 moving with a speed of 20ms−1 hits an ice block (0∘C) of 990g kept at rest on a frictionless floor and gets embedded in it. If ice takes 50% of K.E, the amount of ice melted (in grams) approximately is (J=4.2J/cal) (latent heat of ice=80cal/g)
View SolutionAssertion (A) & Reason (R ) Type Questions
(A): During a free expansion of an ideal gas workdone is zero
(R): Workdone against vacuum is zero
View SolutionA constant pressure gas thermometer has a volume of 200cc, 273.2cc and 525 .1cc at 0°C, 100°C and boiling point of sulphur respectively. The resistance possessed by a platinum wire at the same temperatures as above are 2.000Ω,2.778Ωand5.280Ω respectively. Estimate the boiling point of sulphur on the basis of the two sets of observations.
View SolutionIf a temperature scale defines the freezing point of water as 100∘C and the boiling as 0∘ what temperature on the scale corresponds to 25∘ C ?
View Solutioncalculate the heat absorbed by a system in going through the cyclic process shown in ,
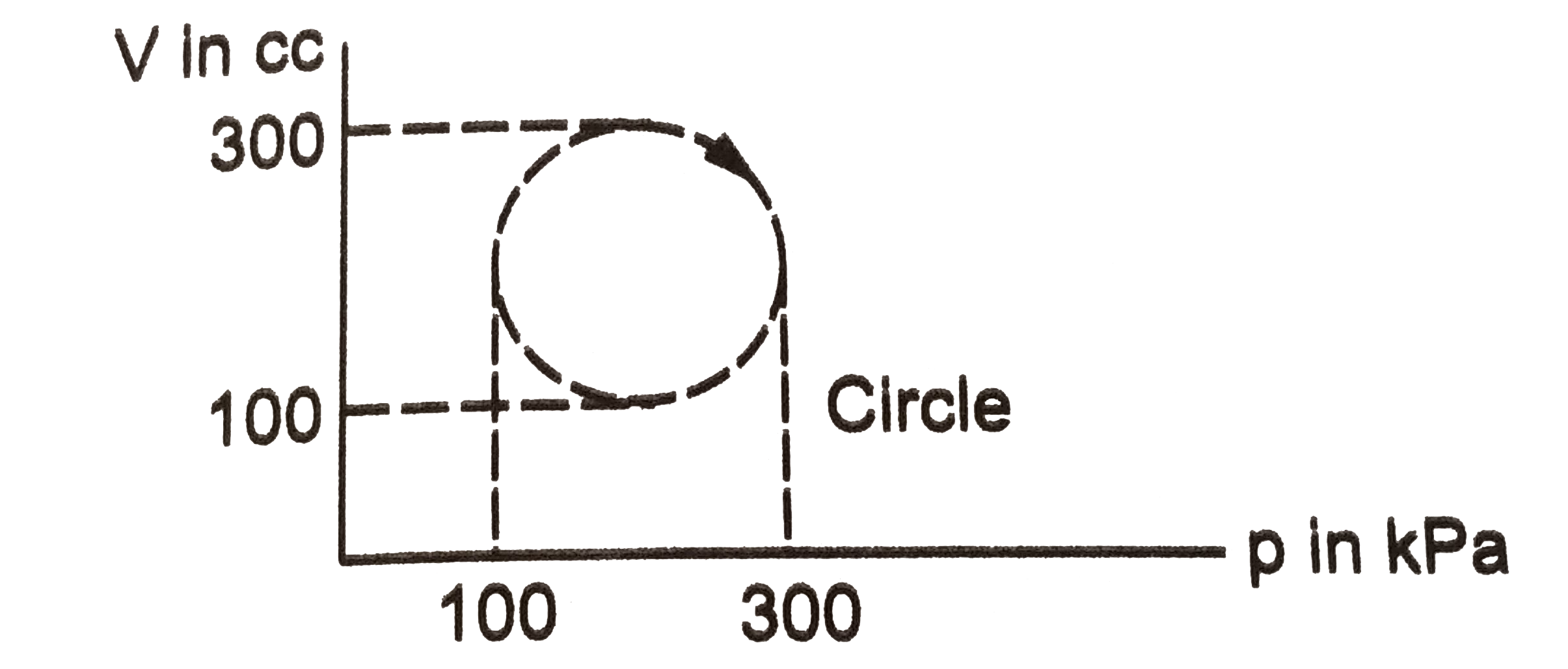
View SolutionHeat energy absorbed by a system in going through a cyclic process shown in figure is
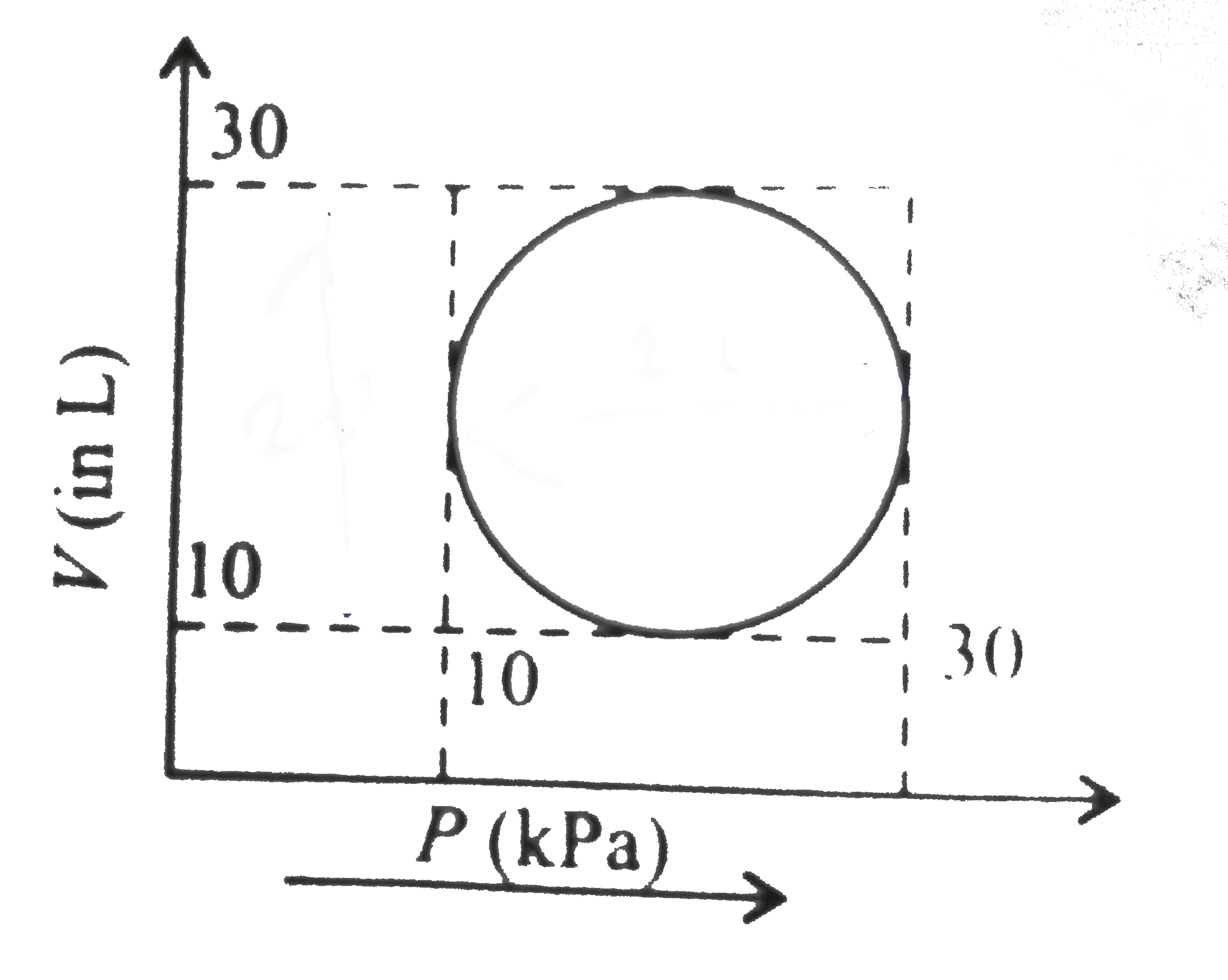
View SolutionHeat energy absorbed by a system in going through a cyclic process shown in Fig.
View SolutionHeat energy abosrbed by a system in going through a cyclic process shown in figure is
View Solution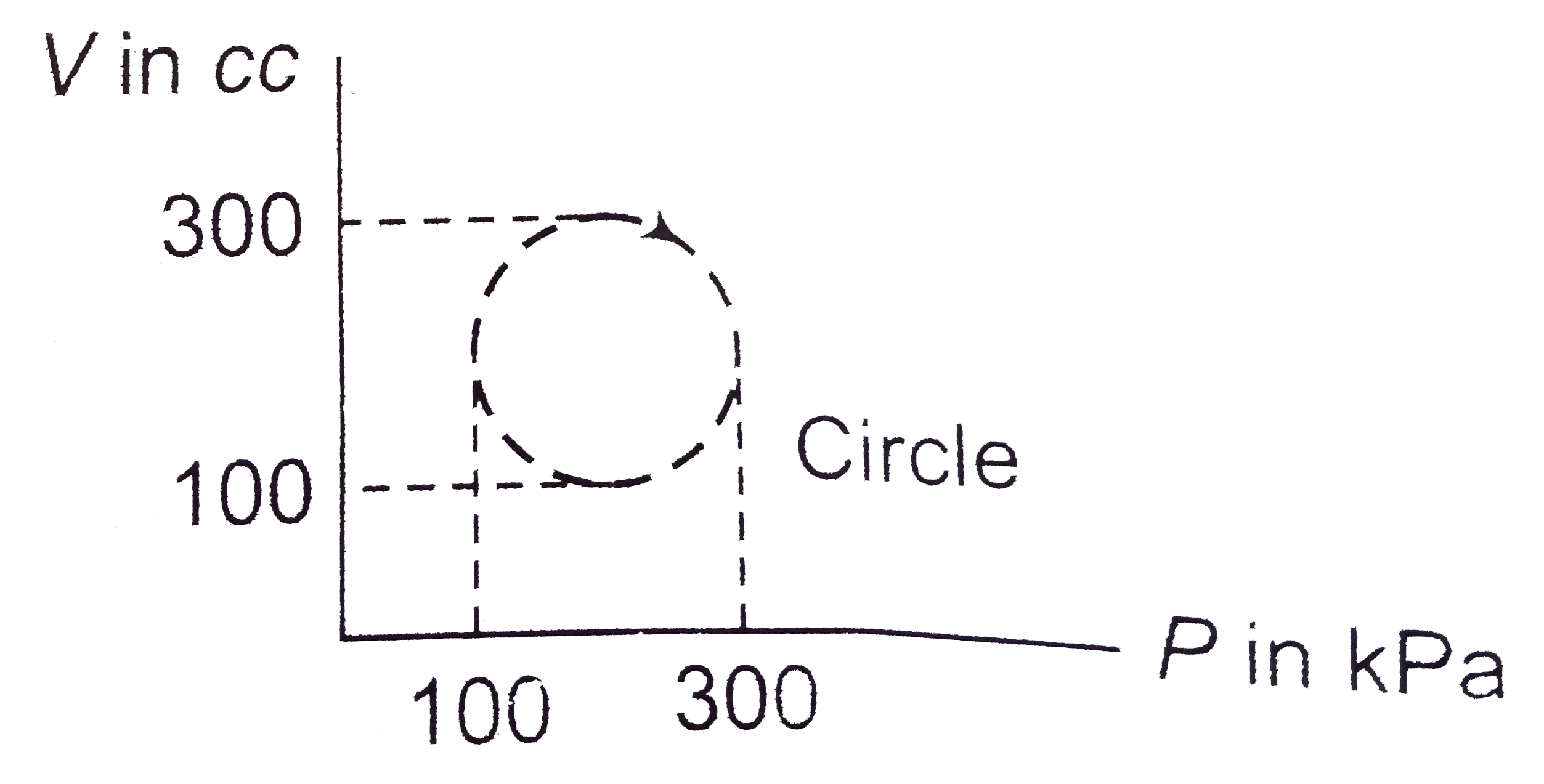
Calculate the heat rejected by a system in going through the cyclic process shown in figure.
View SolutionThe heat energy absorbed by a system in going through a cyclic process shown in figure is
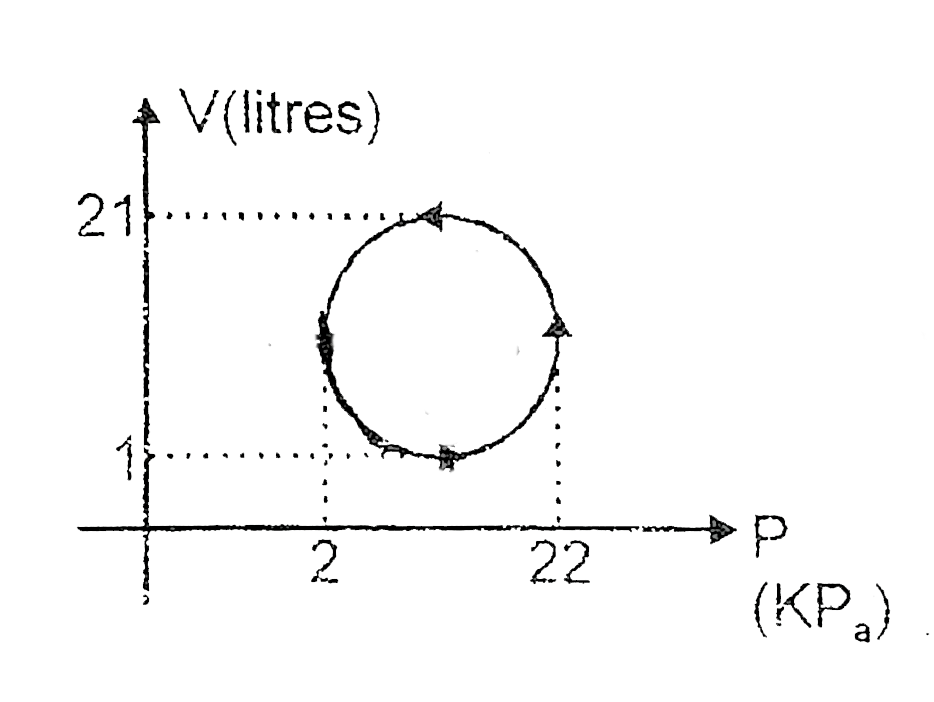
View SolutionCalculate the heat absorbed by the system is going through the process shown in figure
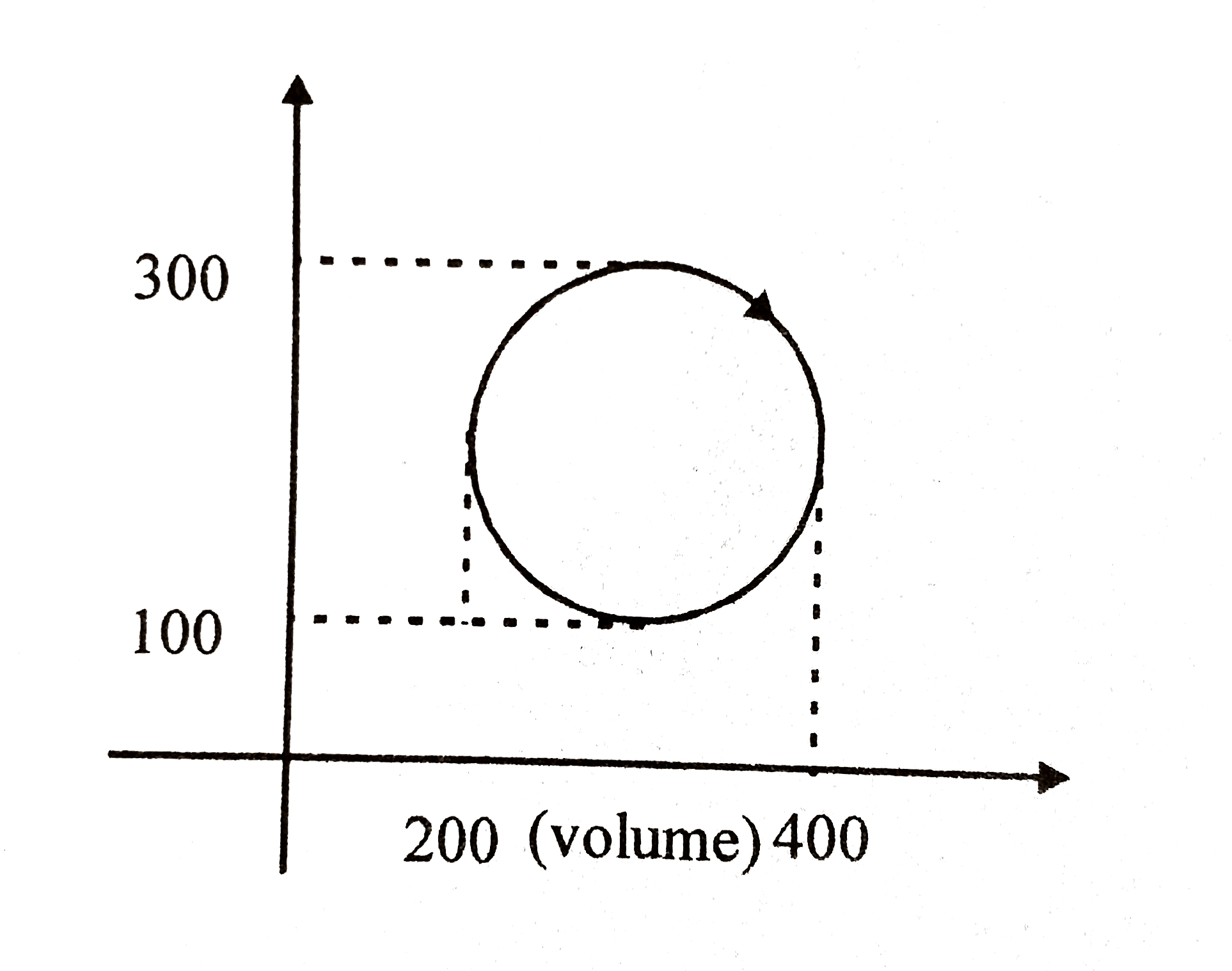
View SolutionThe heat absorbed by the system in going through the cyclic process as shown in figure is
View SolutionHeat absorbed by a system is going through a cyclic process shown in figure is
View Solutioncalculate the heat absorbed by a system in going through the cyclic process shown in ,

View SolutionHeat energy absorbed by a system in going through a cyclic process shown in figure is

View Solution
