Which of the following reduction reaction CANNOT be carried out with coke?
Fe2O3→Fe
Cu2O→Cu
ZnO→Zn
Al2O3→Al
To determine which of the following reduction reactions cannot be carried out with coke, we need to analyze the reduction potential of each metal oxide listed in the options: ZnO, Al2O3, Fe2O3, and Cu2O.
1. Understanding Coke as a Reducing Agent:
- Coke (carbon) is commonly used as a reducing agent in metallurgical processes. It can reduce metal oxides to their corresponding metals, but its effectiveness depends on the stability of the oxide and the affinity of the metal for oxygen.
2. Analyzing Each Metal Oxide:
- ZnO (Zinc Oxide): Zinc oxide can be reduced by coke to produce zinc metal. The reaction is:
ZnO+C→Zn+CO
- Fe2O3 (Iron(III) Oxide): Iron(III) oxide can also be reduced by coke to produce iron metal. The reaction is:
Fe2O3+3C→2Fe+3CO
- Cu2O (Copper(I) Oxide): Copper(I) oxide can be reduced by coke to produce copper metal. The reaction is:
Cu2O+C→2Cu+CO
- Al2O3 (Aluminum Oxide): Aluminum oxide is a very stable compound and cannot be reduced by coke. The reduction of aluminum oxide requires a more powerful reducing agent, typically achieved through electrolysis rather than carbon reduction. The reaction would be:
Al2O3+C→Al+CO
However, this reaction is highly non-spontaneous and does not occur with coke.
3. Conclusion:
- Among the options given, Al2O3 cannot be reduced by coke due to its strong ionic bonds and the high stability of aluminum oxide. Therefore, the correct answer is Al2O3.
Final Answer:
Al2O3 cannot be reduced by coke.
- Coke (carbon) is commonly used as a reducing agent in metallurgical processes. It can reduce metal oxides to their corresponding metals, but its effectiveness depends on the stability of the oxide and the affinity of the metal for oxygen.
- ZnO (Zinc Oxide): Zinc oxide can be reduced by coke to produce zinc metal. The reaction is:
ZnO+C→Zn+CO
- Fe2O3 (Iron(III) Oxide): Iron(III) oxide can also be reduced by coke to produce iron metal. The reaction is:
Fe2O3+3C→2Fe+3CO
- Cu2O (Copper(I) Oxide): Copper(I) oxide can be reduced by coke to produce copper metal. The reaction is:
Cu2O+C→2Cu+CO
- Al2O3 (Aluminum Oxide): Aluminum oxide is a very stable compound and cannot be reduced by coke. The reduction of aluminum oxide requires a more powerful reducing agent, typically achieved through electrolysis rather than carbon reduction. The reaction would be:
Al2O3+C→Al+CO
However, this reaction is highly non-spontaneous and does not occur with coke.
- Among the options given, Al2O3 cannot be reduced by coke due to its strong ionic bonds and the high stability of aluminum oxide. Therefore, the correct answer is Al2O3.
Al2O3 cannot be reduced by coke.
Similar Questions
Knowledge Check
Which one of the following is a reduction reaction
A2Mg(s)+O2→2Mg(s)BS(s)+O2(g)→SO2(g)C2HgO(s)→2Hg(l)+O2(g)DMg(s)+S(s)→MgS(s)Which of the following reactions cannot occur ?
A2AgNO3(aq)+Fe(s)→Fe(NO3)2(Aq)+2Ag(s)BCuSO4(aq)+Zn(s)→ZnSO4(aq)+Cu(s)CCuSO4(aq)+2Ag(s)→Cu(s)+Ag2SO4(aq)D2AgNO3(aq)+Zn(s)→Zn(NO3)2(aq)+2Ag(s)The reduction:

can be best carried out byAClemmensen's reductionBWolff-Kishner reductionCNaBH4DLiAlH4
Clemmenson reduction is carried out with
View SolutionThe reduction
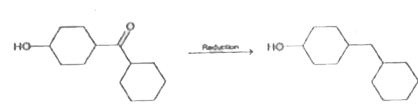
can be best carried out by
View SolutionWhich of the following reaction is oxidation- reduction?
View SolutionWhich one of the following an oxidation reduction reaction ?
View SolutionWhich among the following reactions is reversible when carried out in a closed container ?
View Solution
