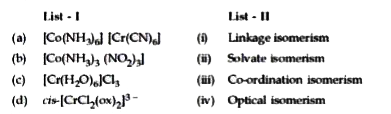Statement I: Sodium hydride can be used as an oxidising agent.
Statement II: The lone pair of electrons on nitrogen in pyridine makes it basic. Choose the CORRECT answer from the options given below:
Statement I is false but statement II is true
Statement I is true but statement II is false
Both statement I and statement Il are false
Both statement I and statement II are true
To solve the problem, we need to analyze both statements provided in the question.
Step 1: Analyze Statement I
Statement I: Sodium hydride can be used as an oxidizing agent.
- Sodium hydride (NaH) consists of sodium ions (Na⁺) and hydride ions (H⁻).
- An oxidizing agent is a substance that gains electrons in a chemical reaction and causes another substance to be oxidized.
- In the case of sodium hydride, the hydride ion (H⁻) acts as a reducing agent because it can donate a hydride ion (H⁻) to another substance, effectively reducing it.
- Therefore, sodium hydride does not act as an oxidizing agent; it is a reducing agent.
Conclusion for Statement I: This statement is incorrect.
Step 2: Analyze Statement II
Statement II: The lone pair of electrons on nitrogen in pyridine makes it basic.
- Pyridine is a six-membered aromatic ring containing one nitrogen atom.
- The nitrogen atom in pyridine has a lone pair of electrons. However, this lone pair is involved in the aromaticity of the ring and is not as available for donation as in other amines.
- Basicity refers to the ability of a substance to accept protons (H⁺ ions). The lone pair on nitrogen in pyridine can indeed be used to accept a proton, making it a base.
- Thus, the presence of the lone pair on nitrogen contributes to the basic nature of pyridine.
Conclusion for Statement II: This statement is correct.
Final Conclusion
- Statement I is incorrect.
- Statement II is correct.
Answer
The correct answer is that Statement I is incorrect and Statement II is correct.
Statement I: Sodium hydride can be used as an oxidizing agent.
- An oxidizing agent is a substance that gains electrons in a chemical reaction and causes another substance to be oxidized.
- In the case of sodium hydride, the hydride ion (H⁻) acts as a reducing agent because it can donate a hydride ion (H⁻) to another substance, effectively reducing it.
- Therefore, sodium hydride does not act as an oxidizing agent; it is a reducing agent.
Statement II: The lone pair of electrons on nitrogen in pyridine makes it basic.
- The nitrogen atom in pyridine has a lone pair of electrons. However, this lone pair is involved in the aromaticity of the ring and is not as available for donation as in other amines.
- Basicity refers to the ability of a substance to accept protons (H⁺ ions). The lone pair on nitrogen in pyridine can indeed be used to accept a proton, making it a base.
- Thus, the presence of the lone pair on nitrogen contributes to the basic nature of pyridine.
- Statement I is incorrect.
- Statement II is correct.
The correct answer is that Statement I is incorrect and Statement II is correct.
Similar Questions
Knowledge Check
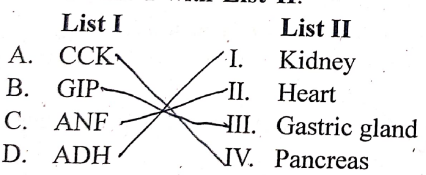
Match List I with List II.
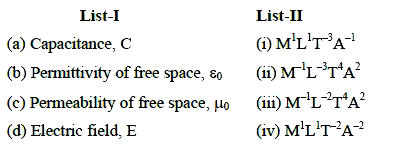
Choose the correct answer from the options given belowA(a) → (iii), (b) → (ii), (c ) → (iv), (d) → (i)B(a) → (iii), (b) → (iv), (c ) → (ii), (d) → (i)C(a) → (iv), (b) → (ii), (c ) → (iii), (d) → (i)D(a) → (iv), (b) → (iii), (c ) → (ii), (d) → (i)Match List-I with List-II.
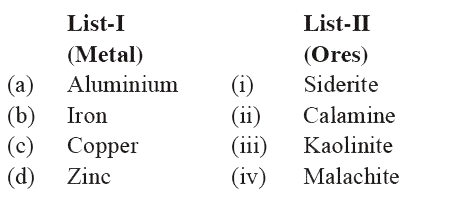
Choose the correct answer from the options given below :A(a)-(iv), (b)-(iii), (c)-(ii), (d)-(i)B(a)-(ii), (b)-(iv), (c)-(i), (d)-(iii)C(a)-(i), (b)-(ii), (c)-(iii), (d)-(iv)D(a)-(iii), (b)-(i), (c)-(iv), (d)-(ii)
Match List I with List II.
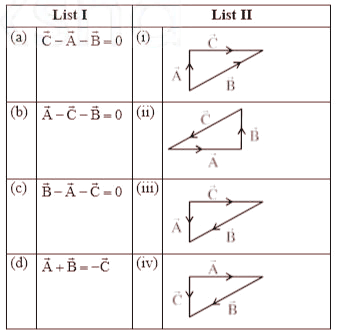
Choose the correct answer from the options given below :
View SolutionMatch List-I with List-II
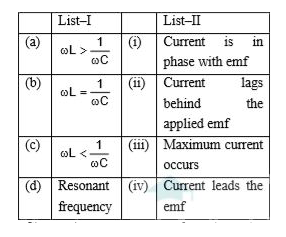
Choose the correct answer from the options given below
View SolutionMatch List-I with List-II

Choose the correct answer from the options given below:
View SolutionMatch List I with List II.

Choose the correct answer from the options given below :
View SolutionMatch List-I with List-II
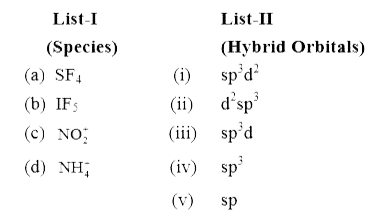
Choose the correct answer from the options given below:
View SolutionMatch List-I with List-II
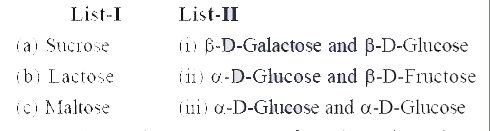
Choose the correct answer from the options given below :
View Solution