One mole of an ideal monoatomaic gas is taken from A to C along the path ABC . The temperature of the gas at A is T0 . For the process ABC :
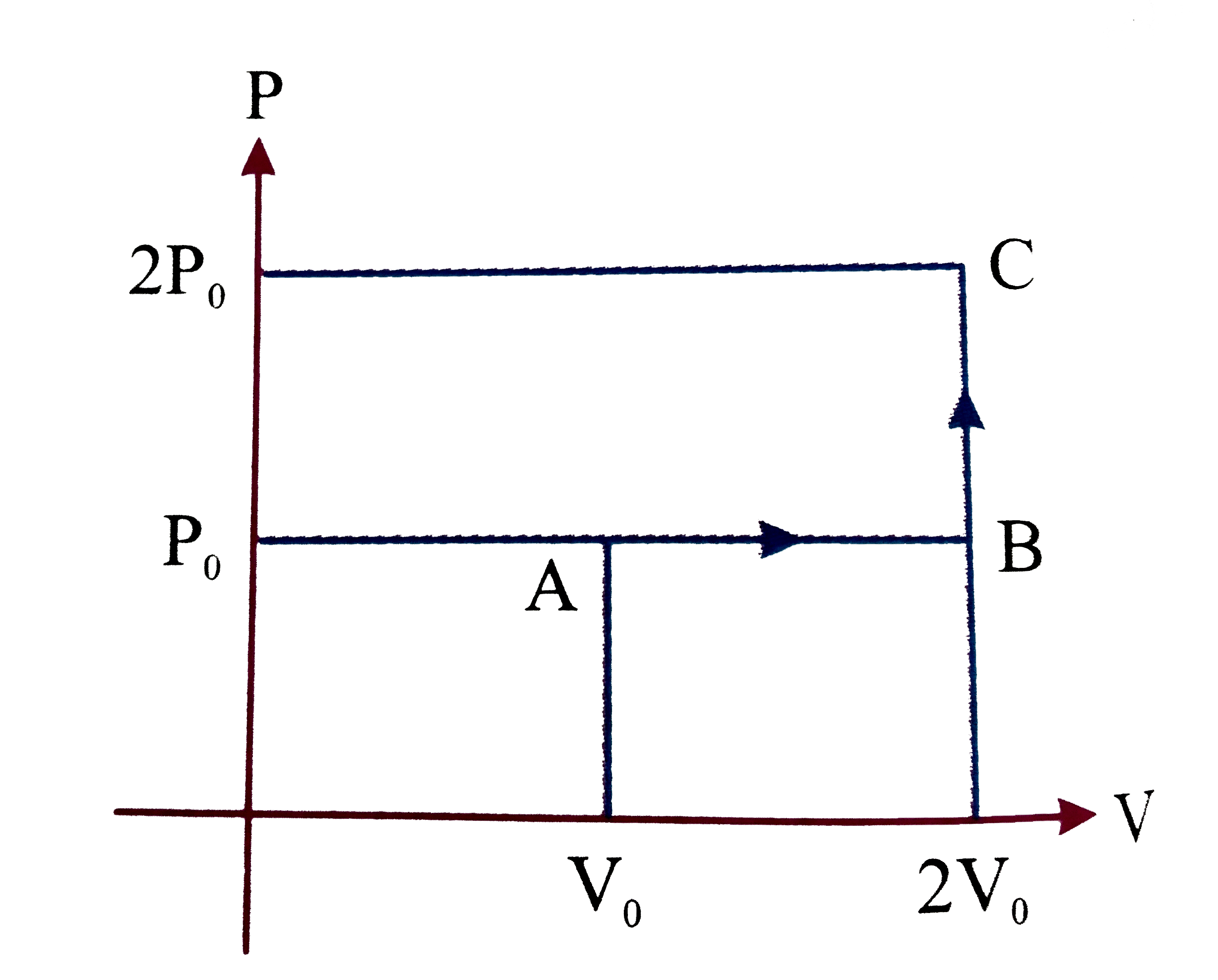
work done by the gas is RT0
change in internal energy of the gas is 112RT0
heal absorbed by the gas is 112RT0
heat absorbed by the gas is 132RT0
The correct Answer is:A, C
Step by step video solution for One mole of an ideal monoatomaic gas is taken from A to C along the path ABC . The temperature of the gas at A is T_(0) . For the process ABC : by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Major parts of communications systems are:
View SolutionThe length of a metal wire is l1 when the tension in it is T1 and is l2 when the tension is T2. Then natural length of the wire is
View SolutionOne mole of a monatomic gas is taken from a point A to another point B along the path ACB . The initial temperature at A is T_(0) . Calculate the heat abosrbed by the gas in the process A rarr C rarr B .
View SolutionOne mole of an ideal monoatomaic gas is taken from A to C along the path ABC . The temperature of the gas at A is T0 . For the process ABC :

View Solution
In the figure n mole of a monoatomic ideal gas undergo the process ABC as shown in the P-V diagram. The process AB is isothermal and BC is isochoric. The temperature of the gas at A is T0 total heat given to the gas during the process ABC is measured to be
Q. Temperature of the gas at C is equal to
View SolutionIn the figure n mole of a monoatomic ideal gas undergo the process ABC as shown in the P-V diagram. The process AB is isothermal and BC is isochoric. The temperature of the gas at A is T_(0) total heat given to the gas during the process ABC is measured to be Q. Heat absorbed by the gas in the process BC
View SolutionIn the figure n mole of a monoatomic ideal gas undergo the process ABC as shown in the P-V diagram. The process AB is isothermal and BC is isochoric. The temperature of the gas at A is T_(0) total heat given to the gas during the process ABC is measured to be Q. The average molar heat capacity of the gas in process ABC is
View SolutionOne mole of an ideal monoatomic gas undergoes a cyclic process as shown in figure. Then the change in the energy in expanding the gas from a to c along the path abc is:
View SolutionTwo moles of a monoatomic gas are taken from a to c, via three paths abc, ac and adc. Work done by the gas in process ac is
View SolutionOne mole of an ideal monoatomic gas is taken along the path ABCA as shown in the PV diagram. The maximum temperature attained by the gas along the path BC is given by :
View Solutionn moles of a diatomic gas has undergone a cyclic process ABC as shown in figure. Temperature at A is T_(0). Find Total heat given to gas ?
View SolutionOne mole of ideal monoatomic gas is taken round the cyclic process as shown in figure. The heat rejected by the gas in the path is ………...and the heat absorbed by the gas in the path AB is ………...........
View SolutionA sample of an ideal gas is taken through the cyclic process abca and shown in the figure. The change in the internal energy of the gas along the path ca is -180J . The gas absorbs 250J of heat along the path ab and 60 J along the path bc. the work done by the gas along the path abc is:
View SolutionOne mole of an ideal monoatomic gas is taken along the path ABCA as shown in the PV diagram. The maximum temperature attained by the gas along the path BC is given by :
View Solutionएक मोल आदर्श गैस का रुद्वोष्म प्रक्रिया द्वारा ताप T1 से T2 तक बढ़ाया जाता है, गैस द्वारा कृत कार्य होगा
View Solution
