In process T∝1V, pressure of the gas increases from p0 to 4p0. Match the following.
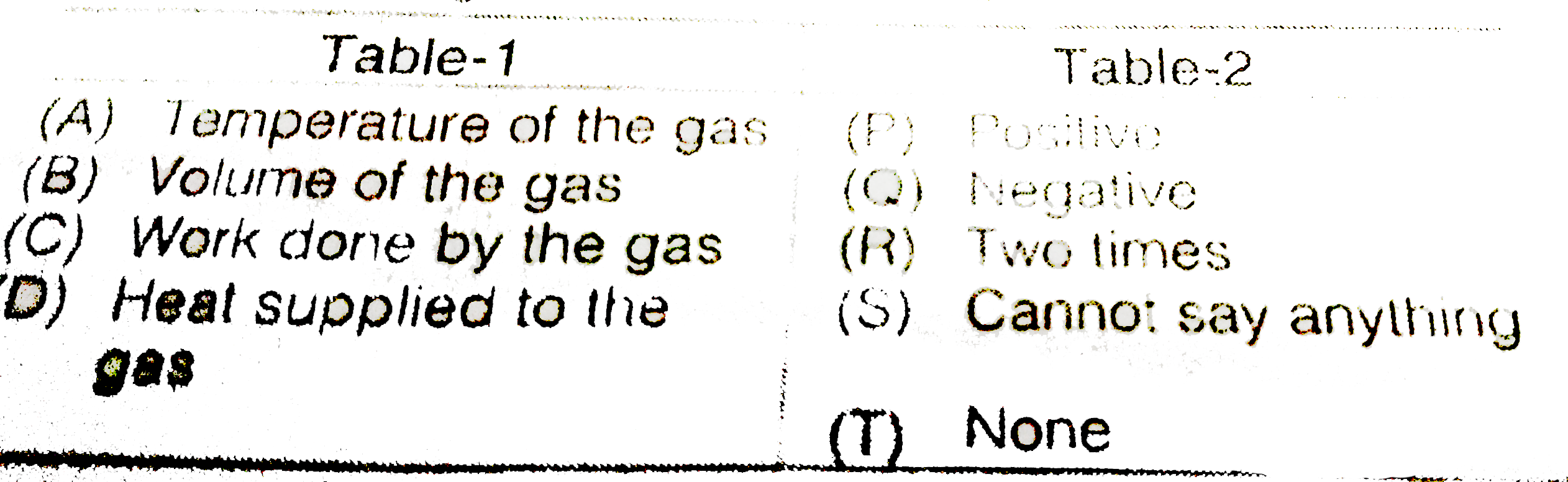
The correct Answer is:(A) R, (B) T, (C) Q, (D) P
Step by step video solution for In process Tprop(1)/(V), pressure of the gas increases from p_(0) to 4p_(0). Match the following. by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
The initial pressure and volume of a given mass of a gas (CpCv=γ) are (p0) and (V0). The gas can exchange heat with surrounding. (a) It is slowly compressed to a volume (V02) and then suddenly compressed to (V04) . Find the final pressure. (b) If the gas is suddenly cpmpressed from the volume (V0→V02) and then slowly compressed to (V04), What will be the final pressure?.
View SolutionA certain mass of an ideal gas is at pressure P1 and volume V1. If is compressed isothermally and then allowed to expand adiabatically untill its pressure returns to P1. The gas is then allowed to expand its original volume. Which of the following P−V graphs are these process correctly shown?
View SolutionA certain mass of an ideal gas is at pressure P1 and volume V1. If is compressed isothermally and then allowed to expand adiabatically untill its pressure returns to P1. The gas is then allowed to expand its original volume. Which of the following P−V graphs are these process correctly shown?
View SolutionOne mole of an ideal monoatomic gas undergoes the process T=T0+4V, where T0 is initial temperature. Find
(i) Heat capacity of gas as function of its volume.
(ii) The amount of heat transferred to gas if its volume increases from V0 to 4V0.
View SolutionThe temperature of n-moles of an ideal gas is increased from T0 to 2T0 through a process p=αT. Find work done in this process.
View Solution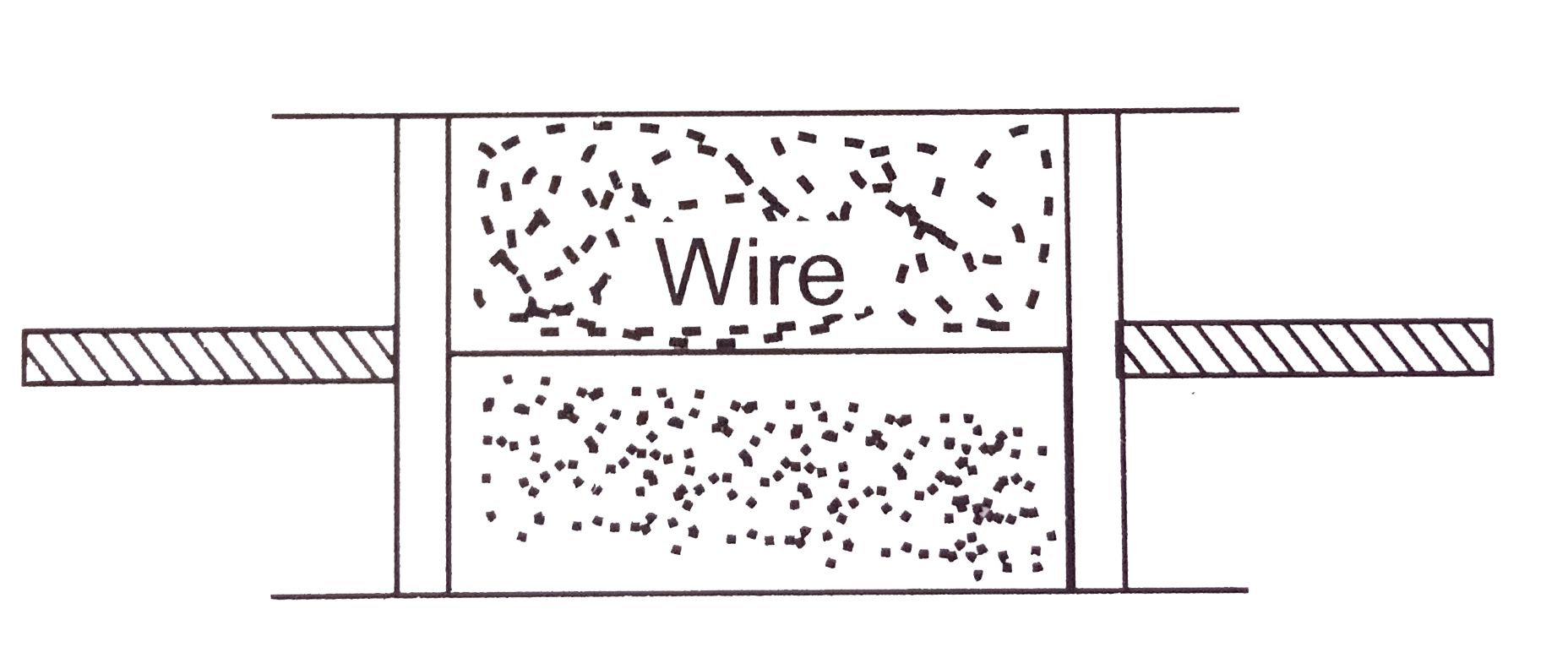
A cylindrical tube of uniform cross-sectional area A is fitted with two air tight frictionless pistons. The pistons are connected to each other by a metallic wire. Initially the pressure of the gas is P0 and temperature is T0, atmospheric pressure is also P0. Now the temperature of the gas is increased to 2T0, the tension in the wire will be
View SolutionThe air tight and smooth piston of a cylindrical vessel are connected with a string as shown. Initially pressure and temperature of the gas are P0 and T0. The atmospheric pressure is also P0. At a later time, tension in the string is 38P0A where A is the cross-sectional are of the cylinder. at this time, the temperature of the gas has become.
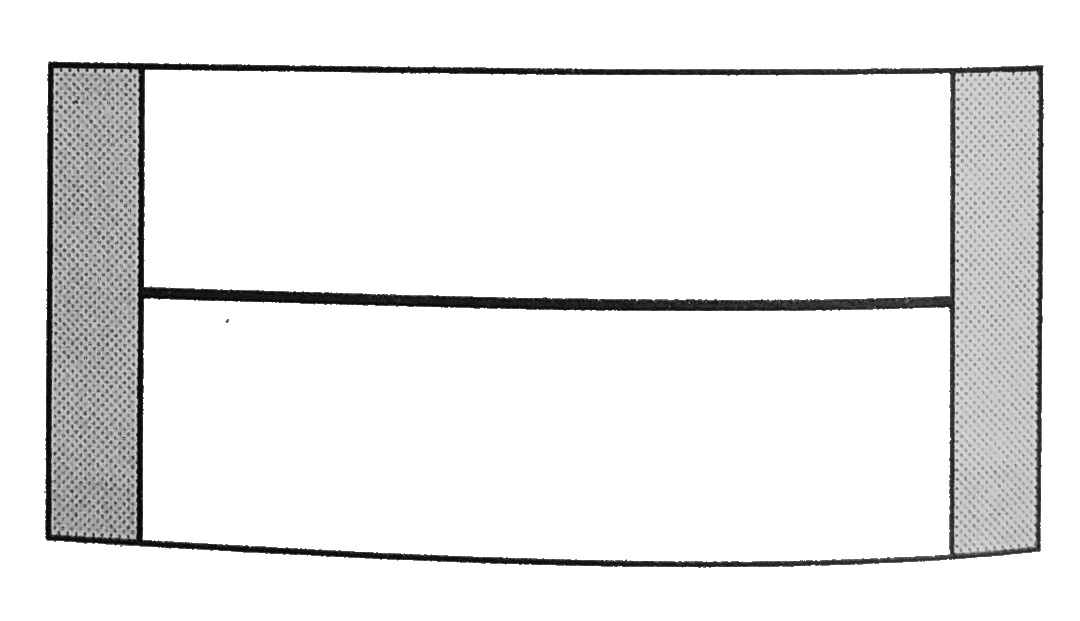
View SolutionDraw the P-T and V-T diagrams of an isochoric process of n moles an ideal gas from pressure P0 volume V0 to pressure 4P0, indicating the pressures and temperatures of the gas in the initial and the final states.
View SolutionAn ideal diatomic gas with CV=5R2 occupies a volume V1 at a pressure P1. The gas undergoes a process in which the pressure is proportional to the volume. At the end of the process the rms speed of the gas molecules has doubled from its initial value.
Heat supplied to the gas in the given process is
View SolutionAn ideal gas is initially at P1,V1 is expands to P2,V2 isothermally and then compressed adiabatically to the same volume V1 and pressure P3. If W is the net work done by the gas in complete process which of the following is true.
View Solution
