The ratio between total acceleration of the electron in singly ionized helium atom and hydrogen atom (both in ground state) is
1
8
4
16
The correct Answer is:B
To find the ratio between the total acceleration of the electron in a singly ionized helium atom and a hydrogen atom in their ground states, we can follow these steps:
1. Understanding Acceleration in Atoms:
The total acceleration a of an electron in an atom can be expressed in terms of its velocity V and radius r of the orbit:
a∝V2r
2. Relating Velocity and Radius:
For an electron in an atom, the velocity V is proportional to the atomic number Z divided by the principal quantum number n:
V∝Zn
The radius r of the orbit is proportional to n2:
r∝n2
3. Substituting into the Acceleration Formula:
Substituting the expressions for V and r into the acceleration formula gives:
a∝(Zn)2n2=Z2n4
4. Finding the Proportionality for Acceleration:
Therefore, we can express the acceleration as:
a∝Z2n4
5. Considering Ground State:
For the ground state, the principal quantum number n=1. Thus, the acceleration becomes:
a∝Z2
6. Calculating the Ratio for Helium and Hydrogen:
The atomic number Z for hydrogen is 1 and for singly ionized helium (He+) is 2. Therefore, the ratio of the accelerations aHe to aH is:
aHeaH=Z2HeZ2H=2212=41=4
Final Answer:
The ratio between the total acceleration of the electron in singly ionized helium atom and hydrogen atom (both in ground state) is 4.
The total acceleration a of an electron in an atom can be expressed in terms of its velocity V and radius r of the orbit:
a∝V2r
For an electron in an atom, the velocity V is proportional to the atomic number Z divided by the principal quantum number n:
V∝Zn
The radius r of the orbit is proportional to n2:
r∝n2
Substituting the expressions for V and r into the acceleration formula gives:
a∝(Zn)2n2=Z2n4
Therefore, we can express the acceleration as:
a∝Z2n4
For the ground state, the principal quantum number n=1. Thus, the acceleration becomes:
a∝Z2
The atomic number Z for hydrogen is 1 and for singly ionized helium (He+) is 2. Therefore, the ratio of the accelerations aHe to aH is:
aHeaH=Z2HeZ2H=2212=41=4
The ratio between the total acceleration of the electron in singly ionized helium atom and hydrogen atom (both in ground state) is 4.
Topper's Solved these Questions
- MODERN PHYSICSBook:DC PANDEY ENGLISHChapter:MODERN PHYSICSExercise:for JEE Advanced (More than One Options is Correct )
Explore 1 Video
- MODERN PHYSICSBook:DC PANDEY ENGLISHChapter:MODERN PHYSICSExercise:Metch the column
Explore 6 Videos
- MODERN PHYSICSBook:DC PANDEY ENGLISHChapter:MODERN PHYSICSExercise:Integer Type Questions
Explore 17 Videos
- MAGNETISM AND MATTERBook:DC PANDEY ENGLISHChapter:MAGNETISM AND MATTERExercise:Medical gallery
Explore 1 Video
- MODERN PHYSICS - 1Book:DC PANDEY ENGLISHChapter:MODERN PHYSICS - 1Exercise:Level 2 Subjective
Explore 23 Videos
Similar Questions
The wavelengths of Kα X-rays from lead isotopes Pb204,Pb206andPb208 are λ1,λ2andλ3 respectively. Choose the correct alternative.
View Solution√v versus Z graph for dcharacteristic X-rays is as shown in figure. Match the following
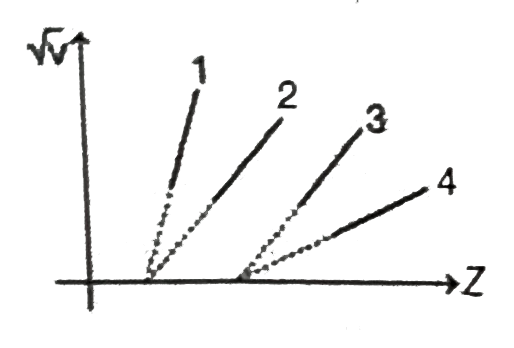
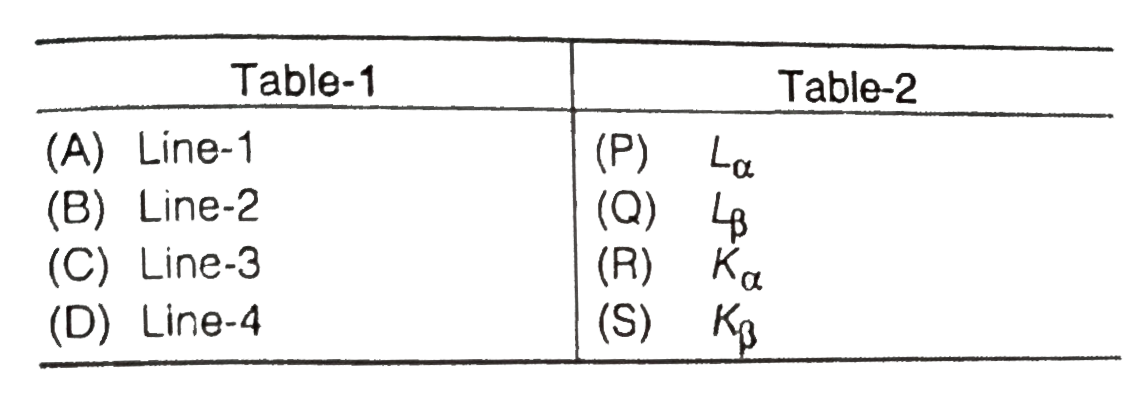
View SolutionThe ratio between acceleration of the electron in singlely ionized helium atom and doublely ionized lithium atom (both in ground state) is (x/27). Find value of x.
View SolutionA bar magnet is hung by a thin cotton thread in a uniform horizontal magnetic field and is in equilibrium state. The energy required to rotate it by 60 is W. Now the torque required to keep the magnet in this new position is
View SolutionThe wavelength for n=3 to n=2 transition of the hydrogen atom is 656.3 nm. What are the wavelength for this same transition in (a) positronium, which consists of an electron and a positron (b) singly ionized helium
View SolutionThe longest wavelength that the singly ionized helium atom in its ground state will obsorb is
View SolutionThe ratio between total acceleration of the electron in singly ionized helium atom and hydrogen atom (both in ground state) is
View SolutionThe ratio between total acceleration of the electron in singly ionized helium atom and hydrogen atom (both in ground state) is
View SolutionThe ratio between acceleration of the electron in singley ionized helium atom and duby ionized lithium aomt (both in ground state) is (x/27). Find value of n.
View SolutionThe ratio between acceleration of the electron in singly ionised helium atom and doubly ionised lithium atom (both in ground state ) is
View SolutionThe energy required to remove the electron from a singly ionized Helium atom is 2.2 times the energy required to remove an electron from Helium atom. The total energy required to ionize the Helium atom ompletelyis:
View SolutionHydrogen ion and singly ionized helium atom are accelerated , from rest , through the same potential difference . The ratio of final speeds of hydrogen and helium ions is close to :
View SolutionThe acceleration of an electron in an ionized helium atom and the ratio of the acceleration of an electron in a hydrogen atom is
View SolutionThe energy required to remove the electron from a singly ionized Helium atom is 2.2 times the energy required to remove an electron from Helium atom. The total energy required to ionize the Helium atom completely is:
View SolutionHydrogen ion and singly ionized helium atom are accelerated , from rest , through the same potential difference . The ratio of final speeds of hydrogen and helium ions is close to :
View Solution
