A cubic vessel (with face horizontal + vetical ) contains an ideal gas at NTP. The vessel is being carried by a rocket which is moving at a speed of 500ms−1 in vertical direction. The pressure of the gas inside the vessel as observed by us on the ground.
remains the same because 500 ms−1 is very much smaller than vrms of the gas
remains the same because motion of the vessel as a whole does not affect the relative motion of the gas molecules and the walls
will increase by a factor equal to (v2rms+(500)2)/v2 where vrms was the original mean squre velocity of the vessel
will be different on the top wall and bottom wall of the vessel
The correct Answer is:B
To solve the problem, we need to analyze the situation of the cubic vessel containing an ideal gas under the influence of motion. The vessel is being carried by a rocket moving vertically at a speed of 500 m/s. We want to determine the pressure of the gas inside the vessel as observed from the ground.
1. Understand the Conditions:
- The vessel is at Normal Temperature and Pressure (NTP), which means the gas is at a standard condition of temperature (0°C or 273.15 K) and pressure (1 atm or 101.3 kPa).
- The rocket is moving vertically at a speed of 500 m/s.
2. Apply the Ideal Gas Law:
- The pressure of an ideal gas can be expressed using the ideal gas equation:
P=nRTV
- Where P is the pressure, n is the number of moles of gas, R is the universal gas constant, T is the temperature, and V is the volume of the gas.
3. Consider the Effects of Motion:
- The vessel is moving with the rocket, but the gas molecules inside the vessel are still colliding with each other and with the walls of the vessel.
- The motion of the vessel does not affect the relative motion of the gas molecules with respect to the walls of the vessel.
4. Collision Dynamics:
- The gas molecules are in constant motion and collide elastically with each other and the walls of the vessel.
- The number of collisions per unit volume remains constant regardless of the vessel's motion.
5. Conclusion on Pressure:
- Since the number of moles, the volume of the vessel, and the temperature remain unchanged, and the collisions are unaffected by the vessel's motion, the pressure inside the vessel remains constant.
- Therefore, the pressure of the gas inside the vessel, as observed from the ground, remains the same as it was at NTP.
6. Final Answer:
- The pressure of the gas inside the vessel remains the same.
- The vessel is at Normal Temperature and Pressure (NTP), which means the gas is at a standard condition of temperature (0°C or 273.15 K) and pressure (1 atm or 101.3 kPa).
- The rocket is moving vertically at a speed of 500 m/s.
- The pressure of an ideal gas can be expressed using the ideal gas equation:
P=nRTV
- Where P is the pressure, n is the number of moles of gas, R is the universal gas constant, T is the temperature, and V is the volume of the gas.
- The vessel is moving with the rocket, but the gas molecules inside the vessel are still colliding with each other and with the walls of the vessel.
- The motion of the vessel does not affect the relative motion of the gas molecules with respect to the walls of the vessel.
- The gas molecules are in constant motion and collide elastically with each other and the walls of the vessel.
- The number of collisions per unit volume remains constant regardless of the vessel's motion.
- Since the number of moles, the volume of the vessel, and the temperature remain unchanged, and the collisions are unaffected by the vessel's motion, the pressure inside the vessel remains constant.
- Therefore, the pressure of the gas inside the vessel, as observed from the ground, remains the same as it was at NTP.
- The pressure of the gas inside the vessel remains the same.
Topper's Solved these Questions
- THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASESBook:DC PANDEY ENGLISHChapter:THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASESExercise:B medical entrance special format questions
Explore 14 Videos
- THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASESBook:DC PANDEY ENGLISHChapter:THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASESExercise:Match the columns
Explore 5 Videos
- THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASESBook:DC PANDEY ENGLISHChapter:THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASESExercise:Check point 14.4
Explore 25 Videos
- SUPERPOSITION OF WAVESBook:DC PANDEY ENGLISHChapter:SUPERPOSITION OF WAVESExercise:Level 2 Subjective
Explore 8 Videos
- THERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASESBook:DC PANDEY ENGLISHChapter:THERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASESExercise:Level 2 Subjective
Explore 9 Videos
Similar Questions
Assertion At the same temperature and pressure, equal volums of all gases gases contain equal numbe of molecules.
Reason In 1 L at NTP total number of molecules are 6.02×1023.
View SolutionFor a monotomic gas at temperature T, match the following columns.
ColumnIColumnII(A)Speed of sound(p)√2RT/M(B)RMS speed of gas molecules(q)√8RT/πM(C)Average speed of gas molecules(r)√3RT/M(D)Most probable speed of gas molecules(s)√5RT/3M
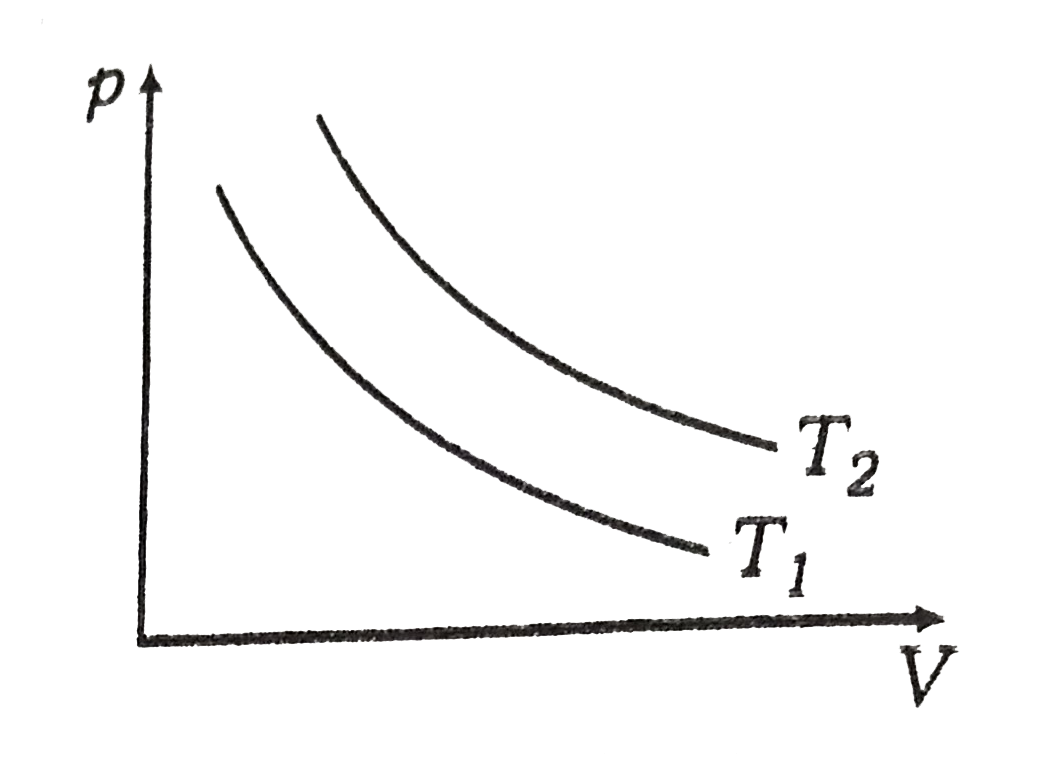
View SolutionThe adjoining fgure shows graph of pressure and volume of a gas at two temperatures T1 and T2 Which one the following is correct
View SolutionThree pieces of string, each of length L, are joined together end-to-end, to make a combined string of length 3L. The first piece of string has mass per unit length μ1, the second piece has mass per unit length μ2=4μ1 and the third piece has mass per unit length μ3=μ1/4 .
(a) If the combined string is under tension F, how much time does it take a transverse wave to travel the entire length 3L? Give your answer in terms of L,F and μ1 .
(b) Does your answer to part (a) depend on the order in which the three piece are joined together? Explain.
View SolutionShow that the volume thermal expansion coefficient for an ideal gas at constant pressure is 1T.
View SolutionCertain amount of an ideal gas is contained in a closed vessel. The vessel is moving with a constant velcity v . The molecular mass of gas is M . The rise in temperature of the gas when the vessel is suddenly stopped is (gamma C_(P) // C_(V))
View SolutionA cubic vessel (with face horizontal + vetical ) contains an ideal gas at NTP. The vessel is being carried by a rocket which is moving at a speed of 500ms−1 in vertical direction. The pressure of the gas inside the vessel as observed by us on the ground.
View SolutionA vessel containing 9 litres of an ideal gas at 760 mm pressure is connected to an evacuated 9 litre vessel. The resultant pressure is
View SolutionThere are two identical vessels containing same quantity of an ideal gas at same pressure vessel A is placed in a train moving with constant speed and the vessel B is placed on the platform in the frame of an observer standing on the platform select the correct statement
View SolutionA cubic vessel (with face horizontal + vetical ) contains an ideal gas at NTP. The vessel is being carried by a rocket which is moving at a speed of 500 ms^(-1) in vertical direction. The pressure of the gas inside the vessel as observed by us on the ground.
View SolutionAt 25^(@)C and 1 atm, a vessel contains 20 L of an ideal gas. If the volume of vessel increased to 40 L, the pressure exerted by gas in vessel will be "______________" .
View SolutionCertain amount of an ideal gas is contained in a closed vessel. The vessel is moving with constant velocity v. The rise in temperature of the gas when the vessel is suddenly stopped is (M is molecular weight of the gas and γ=Cp/Cv)
View SolutionA vessel contains 1 mole of O_2 at a temperature T. The pressure of gas is P. An identical vessel containing 1 mole of the gas at a temperature 2T has pressure of -
View SolutionThe ideal gas is filled at normal heat pressure in a cubic vessel. If this vessel is being carried by a rocket with a velocity of 500 m / s in the vertical direction, what will be the pressure of the gas inside the vessel when viewed from the earth?
View SolutionA cubic vessel (with face horizontal + vetical ) contains an ideal gas at NTP. The vessel is being carried by a rocket which is moving at a speed of 500 ms^(-1) in vertical direction. The pressure of the gas inside the vessel as observed by us on the ground.
View Solution
