Coordination No. |Oxidation State Of Metal
Video Solution
|
Answer
Step by step video solution for Coordination No. |Oxidation State Of Metal by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
In the complex lon [Fe(EDTA)]⊕ the coordination number and oxidation state of central metal ion is:
ACN = 6, ON = +3BCN = 1, ON = -1CCN = 4, ON = +2DCN = 3, ON = +3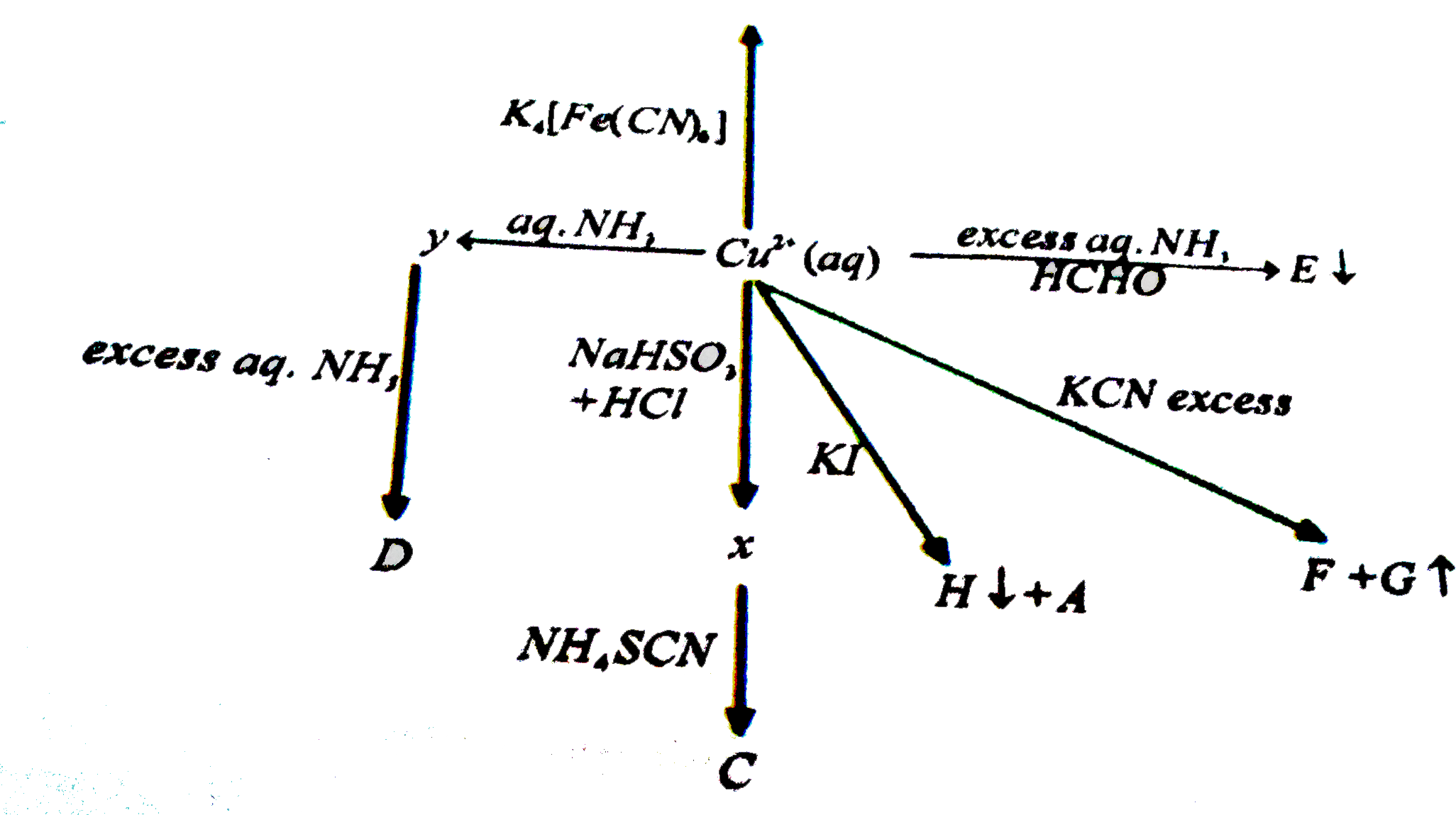
The coordination number and oxidation state of copper in 'F' isA6,+1B4,+2C6,+2D4,+1The compound in which oxidation state of metal is zero-
AFe2(CO)9BNi(CO)4CFe3(CO)9DAll the above
What is the coordination number and the oxidation state of the metal in each of the following complexes ?
(a)[ZrF8]4− ,(b)K3[Cr(C2O4)2Cl2]
View SolutionWhy is the highest oxidation state of a metal exhibited in its oxide or fluoride only ?
View SolutionIn which of the following complexes, oxidation state of metal is zero ?
View SolutionIn which of the following complexes oxidation state of metal is zero
View SolutionThe common oxidation state of alkali metals in the combined state is
View Solution
