Most stable form of meso-2, 3-difluoro-2, 3-butandiol (across C2-C3 bond) is
A

B

C

D

Text Solution
The correct Answer is:C
|
Topper's Solved these Questions
- NOMENCLATURE AND CONFORMATIONS OF ALKANES AND CYCLOALKANESBook:MS CHOUHANChapter:NOMENCLATURE AND CONFORMATIONS OF ALKANES AND CYCLOALKANESExercise:Additional Objective Questions (MCQ)
Explore 1 Video
- NOMENCLATURE AND CONFORMATIONS OF ALKANES AND CYCLOALKANESBook:MS CHOUHANChapter:NOMENCLATURE AND CONFORMATIONS OF ALKANES AND CYCLOALKANESExercise:Additional Objective Questions (MCQ)
Explore 1 Video
- ISOMERISM (STRUCTURAL & STEREOISOMERISM)Book:MS CHOUHANChapter:ISOMERISM (STRUCTURAL & STEREOISOMERISM)Exercise:Subjective Problems
Explore 17 Videos
- PHENOLS AND ARYL HALIDESBook:MS CHOUHANChapter:PHENOLS AND ARYL HALIDESExercise:ADDITIONAL OBJECTIVE QUESTIONS ( SINGLE CORRECT CHOICE TYPE )
Explore 20 Videos
Similar Questions
Knowledge Check
Most stable from of meso-2, 3-diflouro-2, 3-butandiol is :
A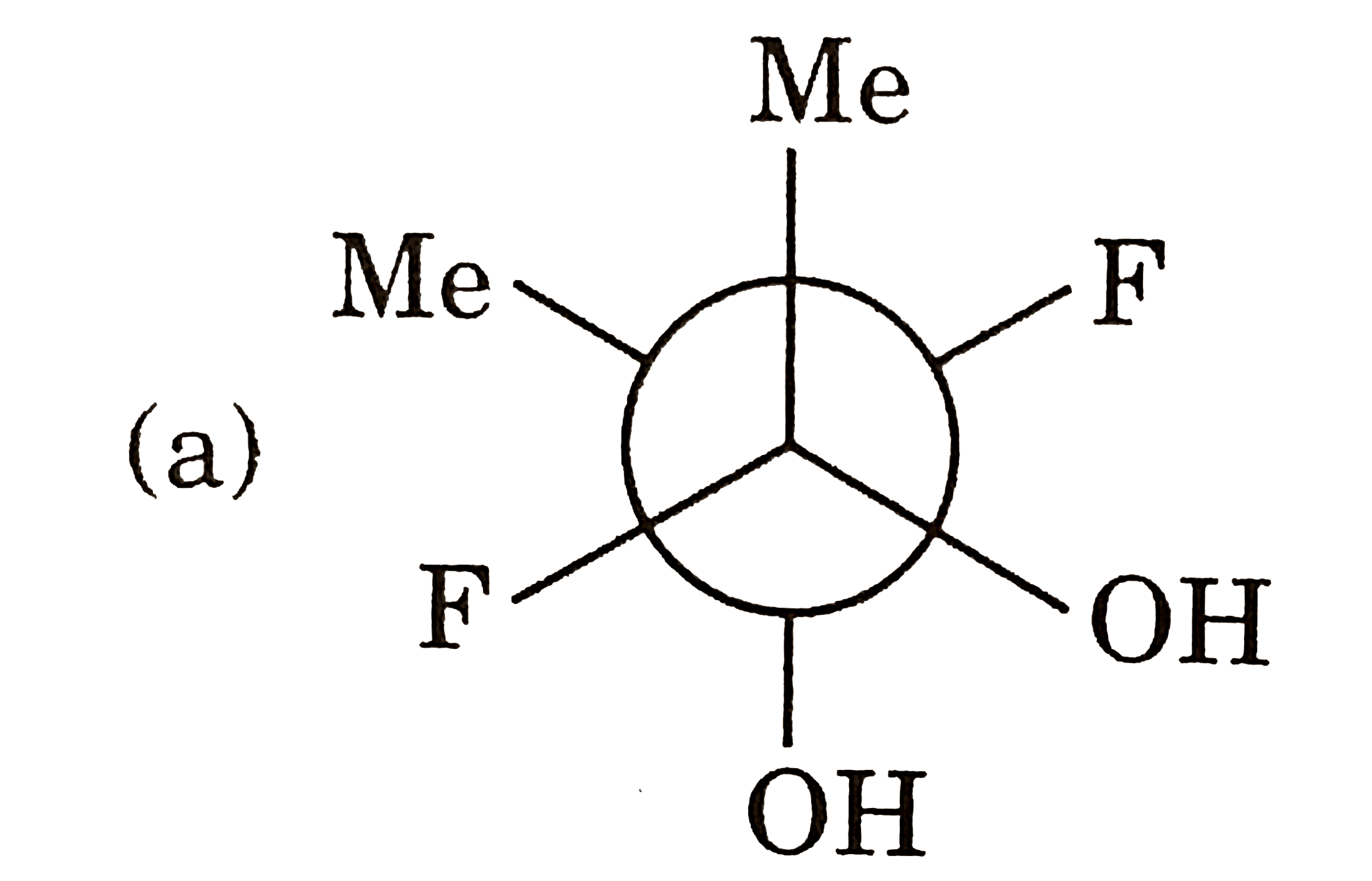 B
B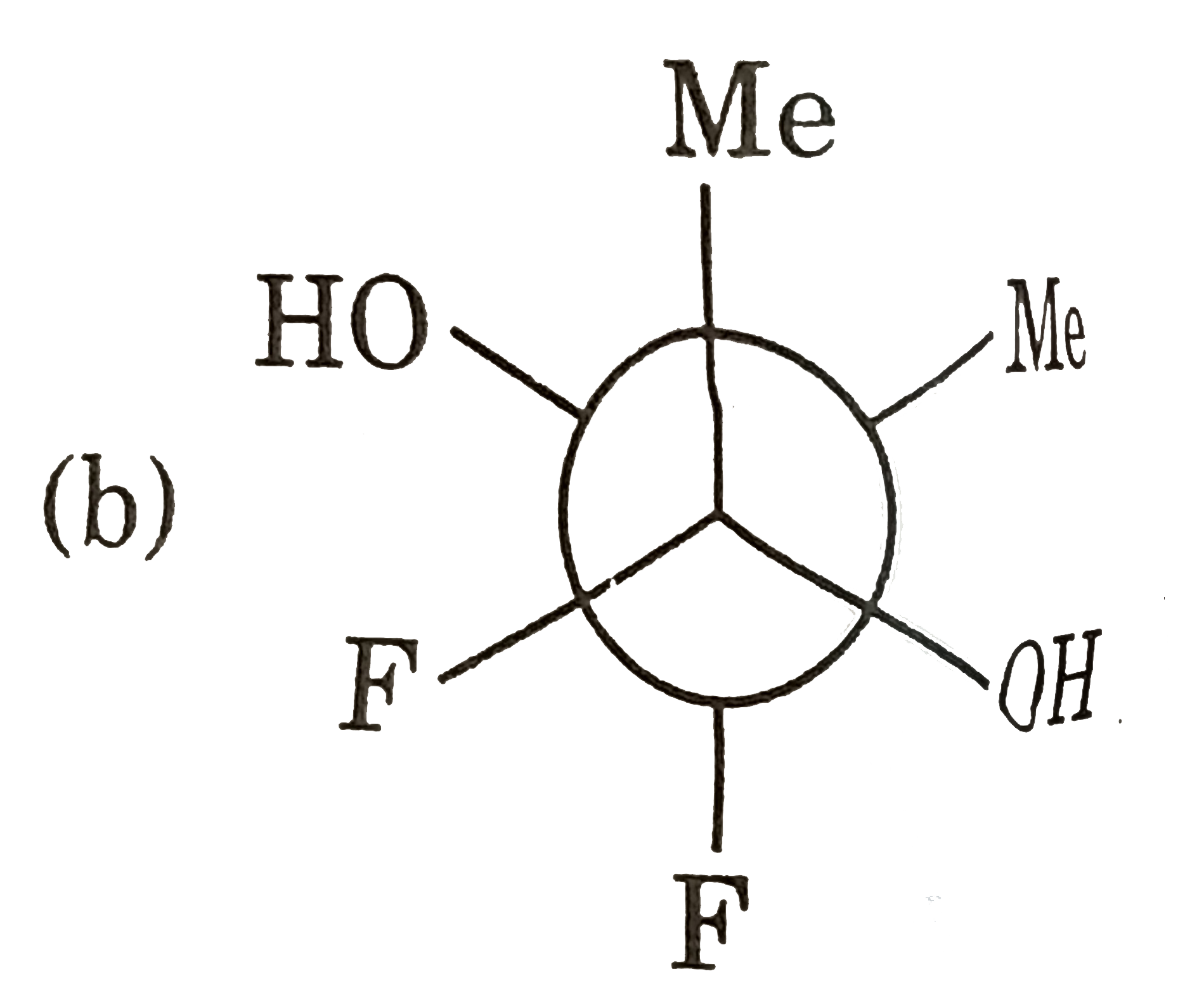 C
C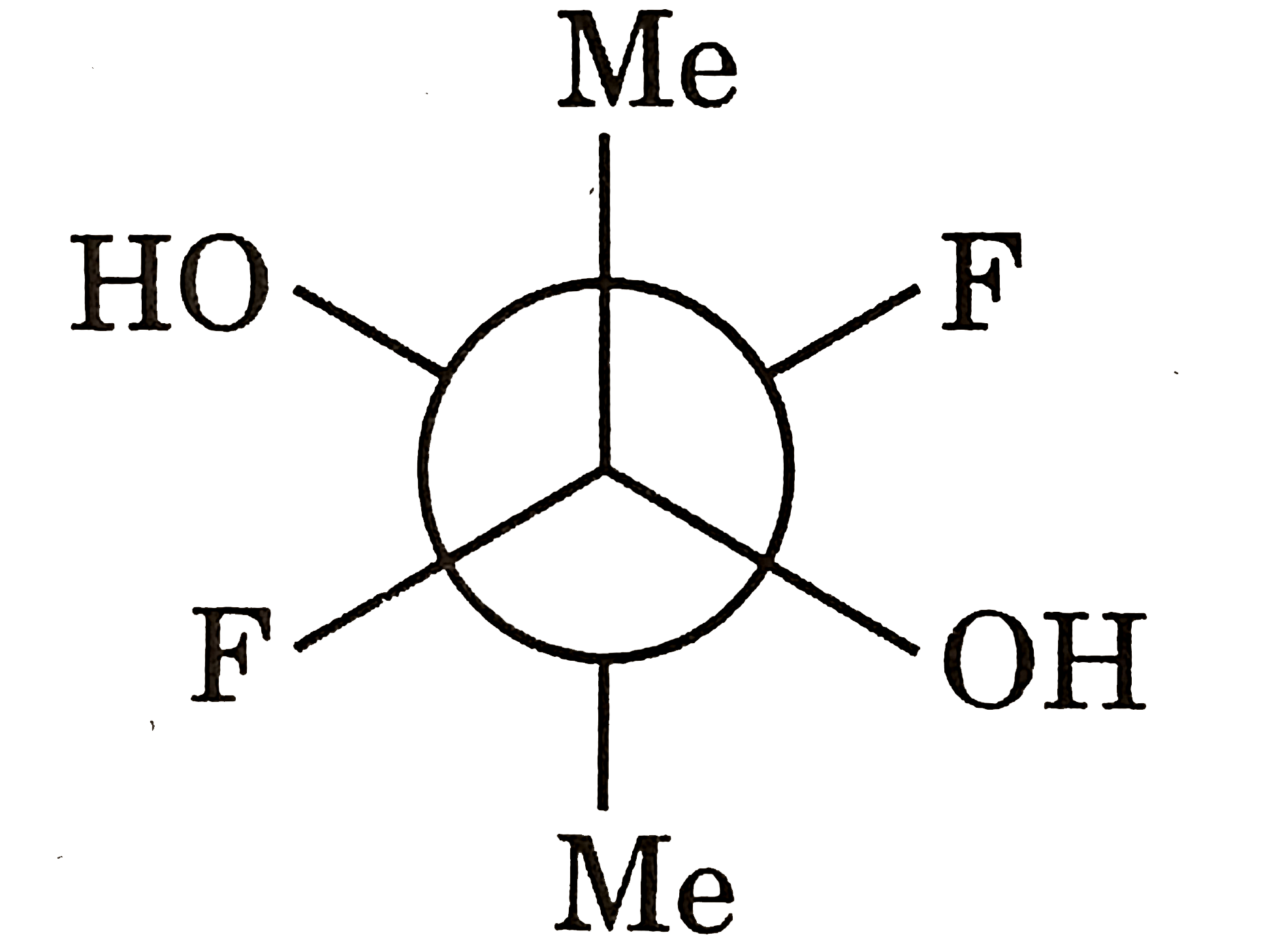 D
D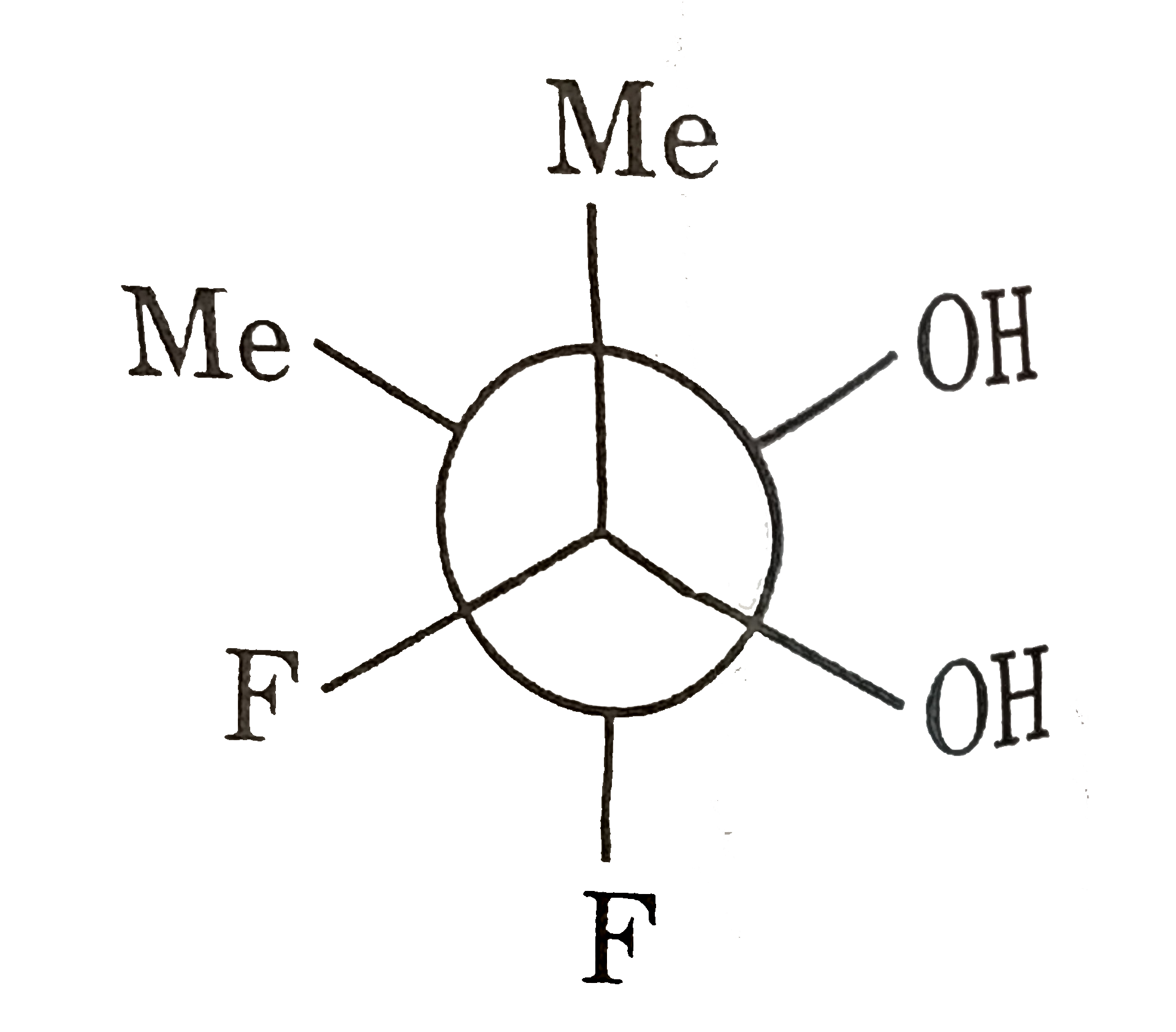
Most stable from of meso-2,3-butandiol is :
A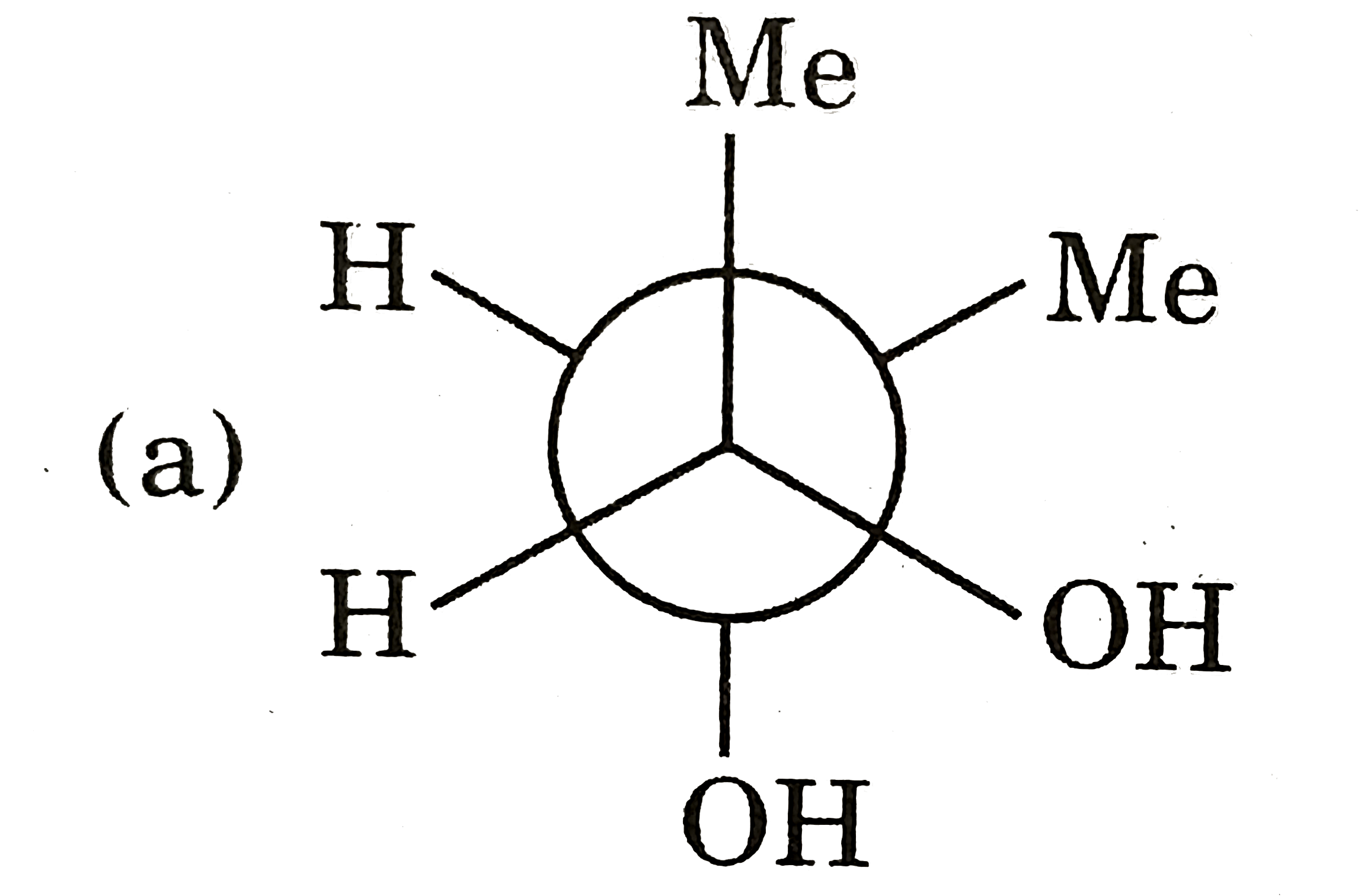 B
B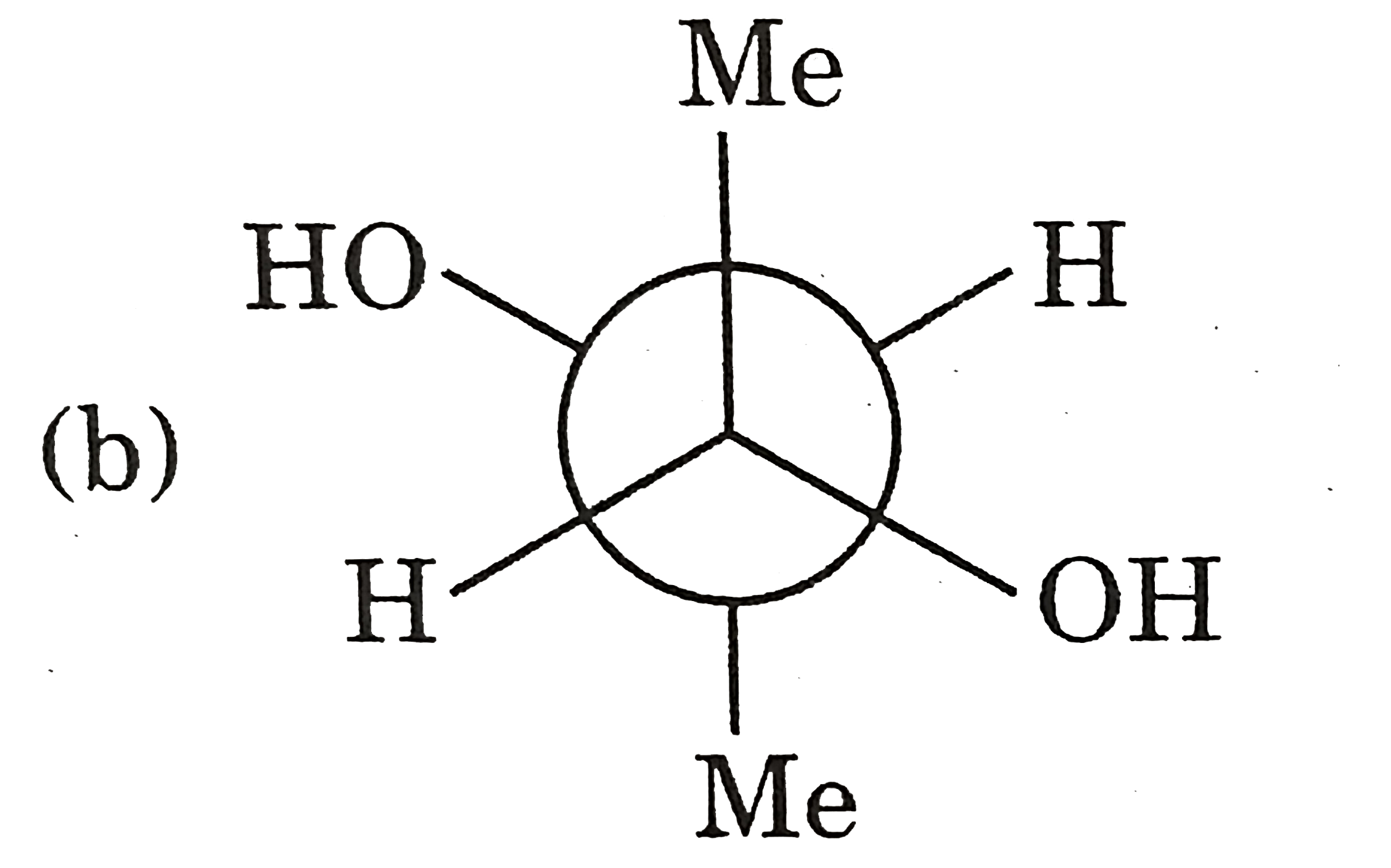 C
C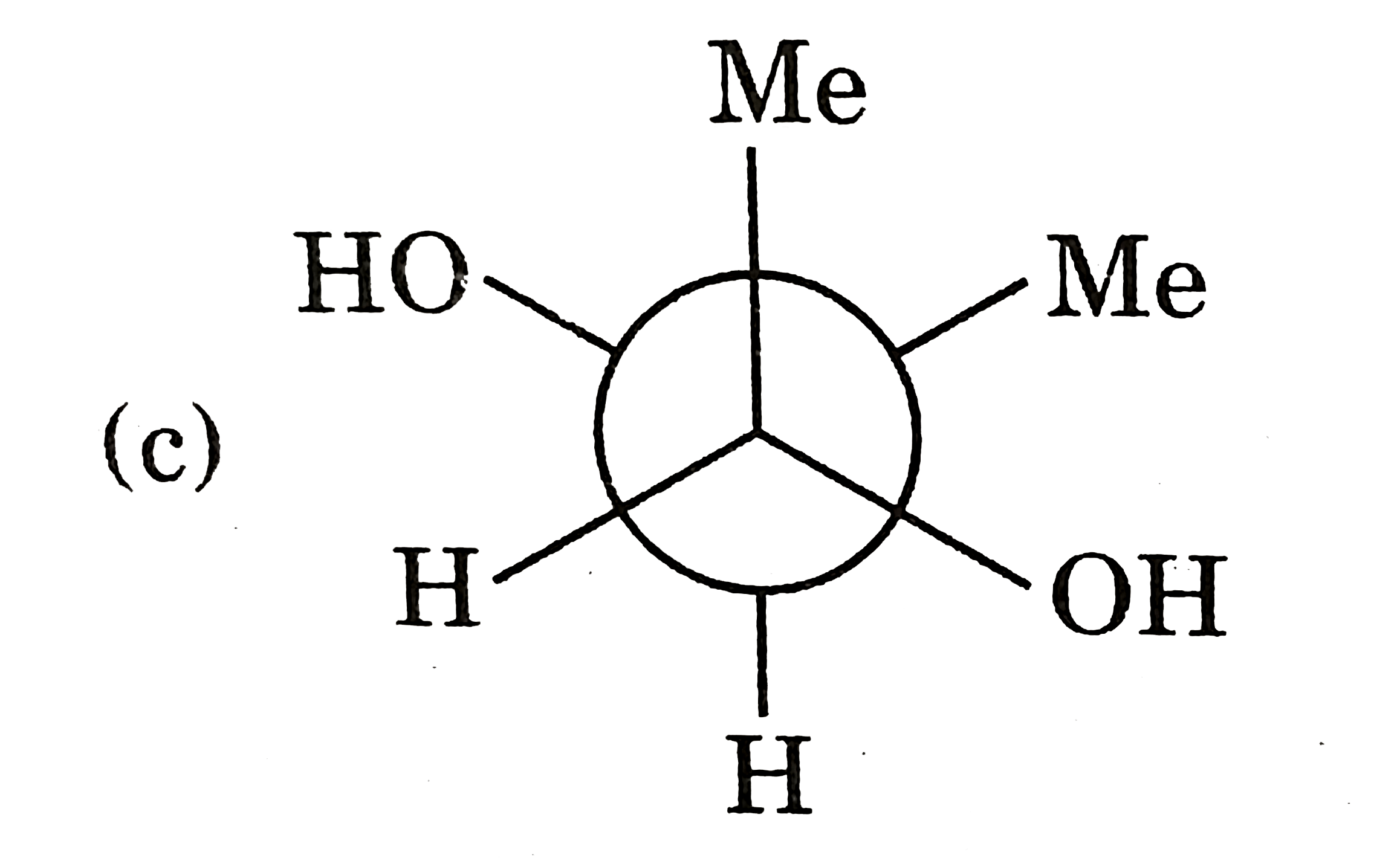 D
D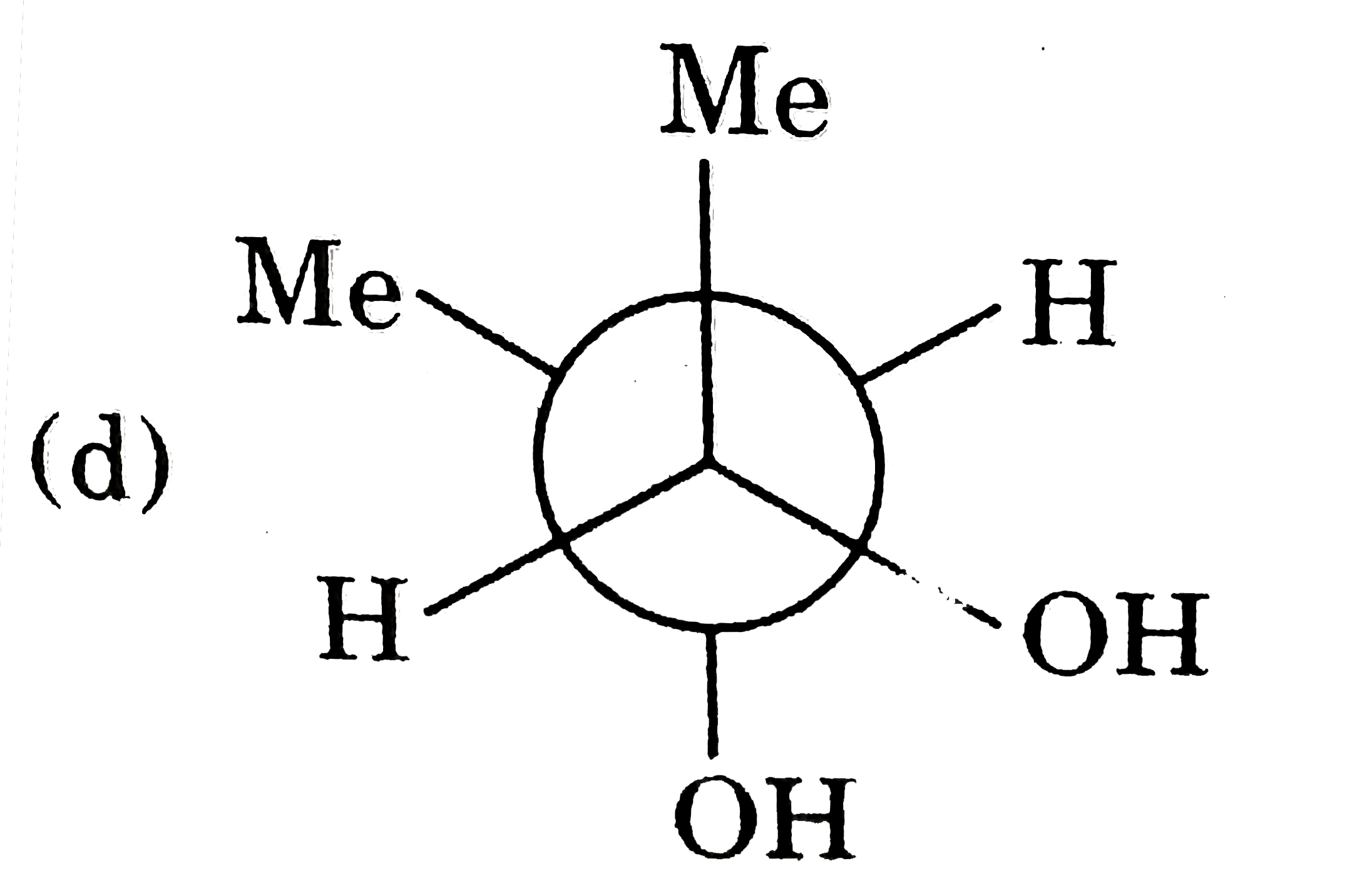
The most stable conformation of 2, 3-dibromobutane is -
A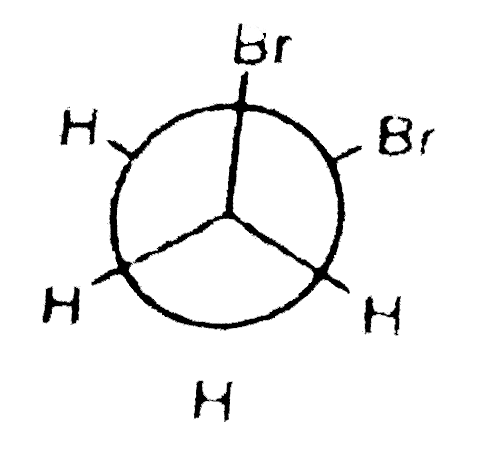 B
B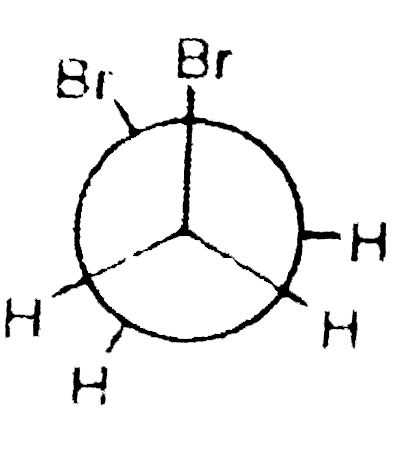 C
C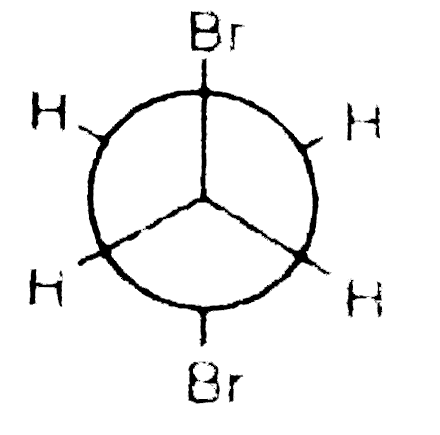 D
D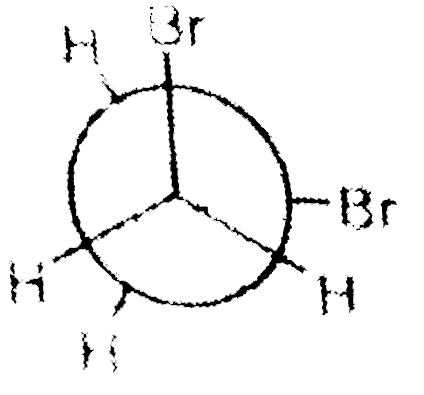
The most stable conformation of 2, 3- dimethyl butane is
View SolutionThe most stable conformation of 2, 3-dimethyl butane is :
View SolutionThe most stable form of meso-tartaric acid is
View SolutionMost stable conformation of n-pentane is :
( Consider C2−C3 bond rotation )
View SolutionThe meso form of 2, 3, 4-pentanetriol is :
View Solution

